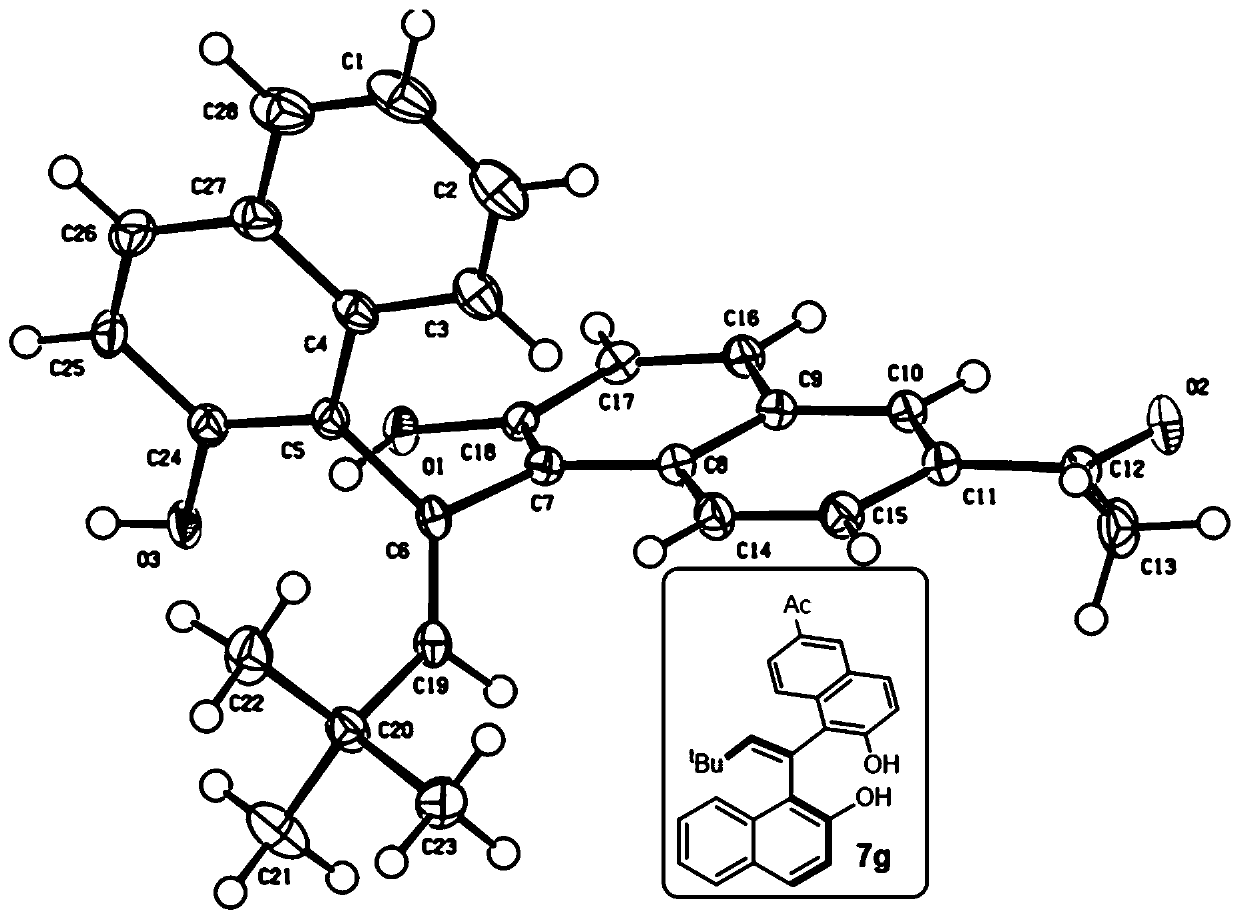
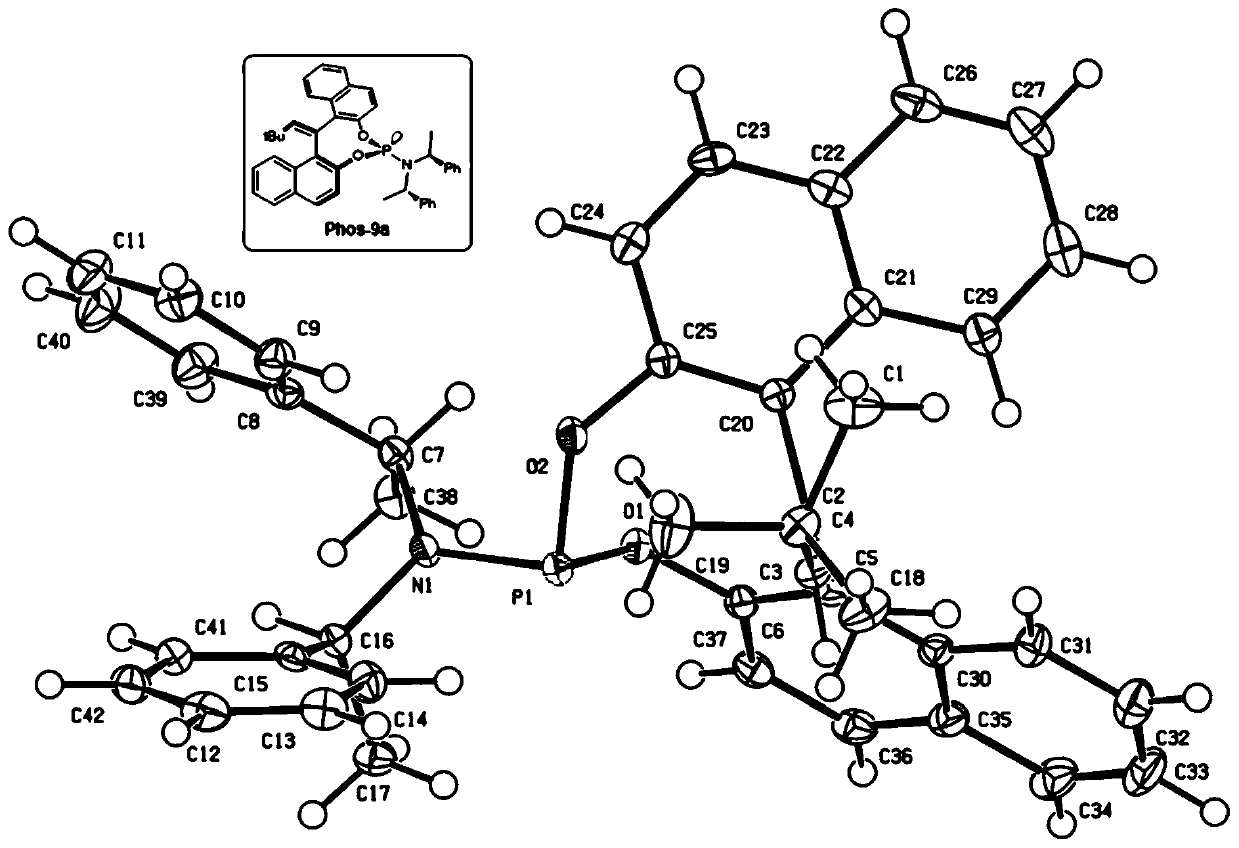
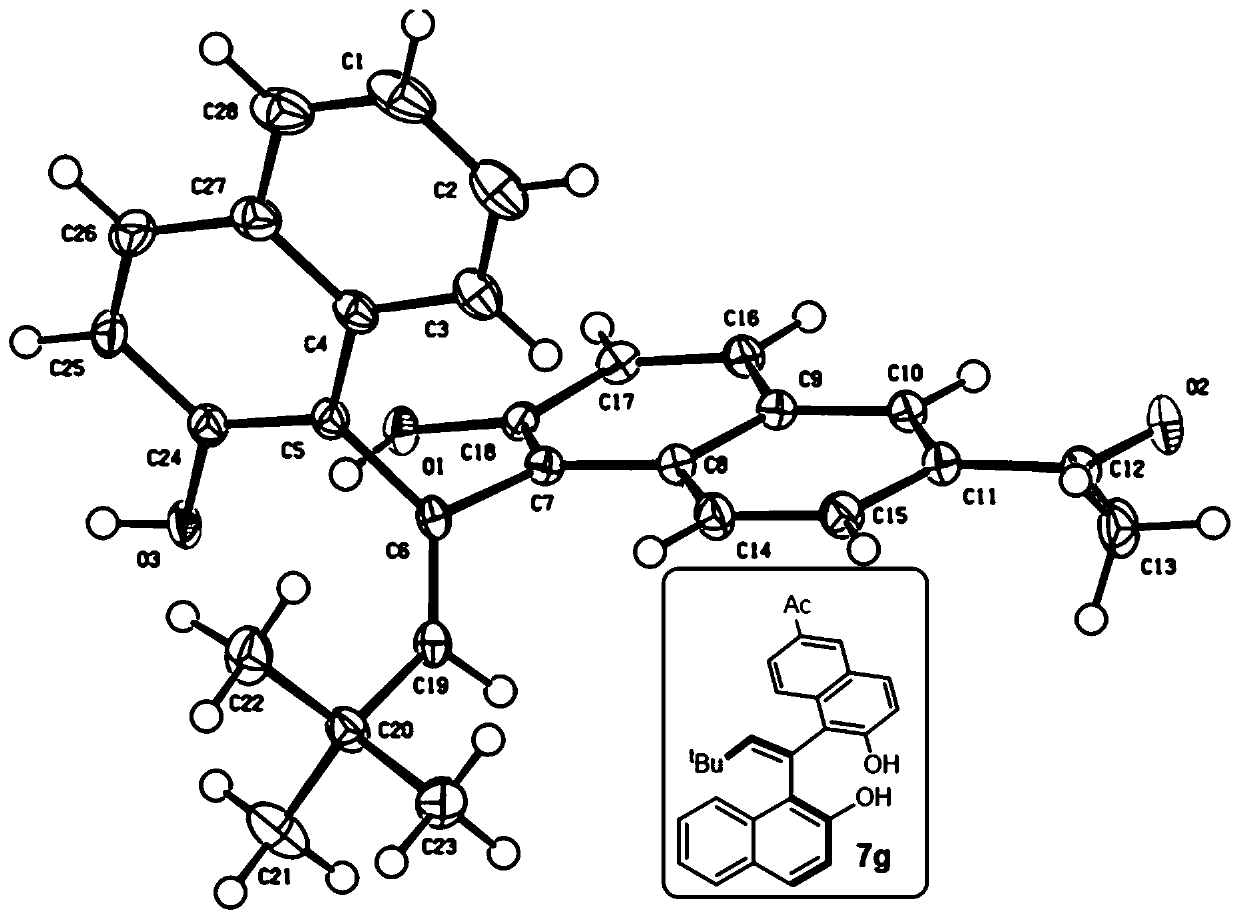
EBINOL axial chiral compound as well as synthesis method and application thereof
A compound and axial chiral technology, which is applied in the field of EBINOL axial chiral compounds and their synthesis, can solve problems such as poor enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Synthesis of substrates
[0077]
[0078] At 0°C, under stirring conditions, to concentrated H 2 SO 4 (0.98mL, 18.0mmol) in MeOH (20mL), 2-naphthol compound J (12.0mmol) were added KI (2.19g, 13.2mmol) and 30% H 2 o 2 (2.72mL, 24.0mmol), the mixture was stirred at 0°C for about 2 hours, after the completion of the reaction was monitored by TLC, the mixture was diluted with water, extracted twice with 50mL DCM, and the combined organic phase was successively washed with 20mL saturated Na 2 S 2 o 3 , 40mL H 2 O and 50mL of brine, washed with Na 2 SO 4 After drying and concentration under reduced pressure, the residue was purified by recrystallization to give the product K.
[0079] Under argon protection, compound K (10.0 mmol), Pd(PPh 3 ) 4 (1.16g, 1.00mmol), CuI (381mg, 2.00mmol) and 40mL of anhydrous THF, then added 3,3-dimethyl-1-butyne (2.46mL, 20.0mmol) and i PR 2 NH (4.20 mL, 30.0 mmol). The Schlenk tube was sealed, and the mixture was stirred at ro...
Embodiment 2
[0105] Screening of reaction conditions: 6a (0.10mmol), 1a (0.15mmol) and catalyst (5mol%) were reacted in 2.0mL solvent for 36 hours.
[0106]
[0107]
[0108]
[0109] b: separation yield; c: determined by HPLC analysis; d: 48 hours of reaction; e: 0.2 mmol of 6a, 0.3 mmol of 1a.
[0110] 1-(3,3-Dimethyl-1-ynyl)-2-naphthol 6a and 2-naphthol 1a were selected as substrates. In the presence of SPINOL phosphoric acid (S)-C1, the reaction proceeded smoothly to 86 % yield, 71% ee afforded axial chiral EBINOL(aR)-7a. Then, phosphoric acid catalysts with different chiral skeletons and substituents were screened, and it was found that both SPINOL phosphoric acid and BINOL phosphoric acid C10 had excellent enantioselectivity, and C3 was the catalyst with the best effect. After screening the solvent, it was found that DCM was the best choice, and after screening the temperature, it was found that the reaction at 10° C. for 48 hours had the best effect (98% ee, 93% yield).
...
Embodiment 3
[0117]
[0118] Following the general synthetic procedure, 7a was obtained as a white solid in 96% yield, 98% ee.
[0119] 1 H NMR (400MHz, acetone-d 6 )δ8.99(brs,2H),8.73(d,J=8.7Hz,1H),8.10(s,1H),7.80(d,J=8.0Hz,1H),7.76(d,J=8.9Hz, 1H), 7.74–7.72(m, 1H), 7.68(d, J=8.8Hz, 1H), 7.59(s, 1H), 7.34(t, J=7.3Hz, 1H), 7.27(d, J=8.8 Hz, 1H), 7.21–7.18(m, 2H), 7.05(d, J=8.8Hz, 1H), 6.17(s, 1H), 1.04(s, 9H). 13 C NMR (101MHz, acetone-d 6 )δ153.27,152.90,152.23,135.58,134.49,130.50,130.24,130.13,129.89,129.62,129.11,127.10,126.88,126.30,125.56,124.62,124.34,123.86,123.49,121.14,119.16,118.44,36.13,29.96。 HRMS (ESI) accurate mass calculation [M-H] - C 26 h 23 o 2 - , m / z: 367.1704, measured value: 367.1699. IR(KBr,cm -1 ) 3464, 3364, 2961, 1622, 1516, 1341, 1269, 1200, 816, 750. M.P. 226-228°C. (c=0.4, CHCl 3 ). HPLC conditions: HPLC DAICEL CHIRALPAK ID, n-hexane / isopropanol=95 / 5, 0.5mL / min, λ=230nm, t R (minor) = 10.0min,t R (major) = 11.6 min, ee = 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com