Repaglinide composite medicine and preparation method thereof
A technology for drugs and adhesives, applied in the field of medicine, can solve the problems of the use of repaglinide, increase hypoglycemia, drug waste, etc., and achieve the effects of reducing production costs, accelerating metabolism, and preventing the level from rising too fast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
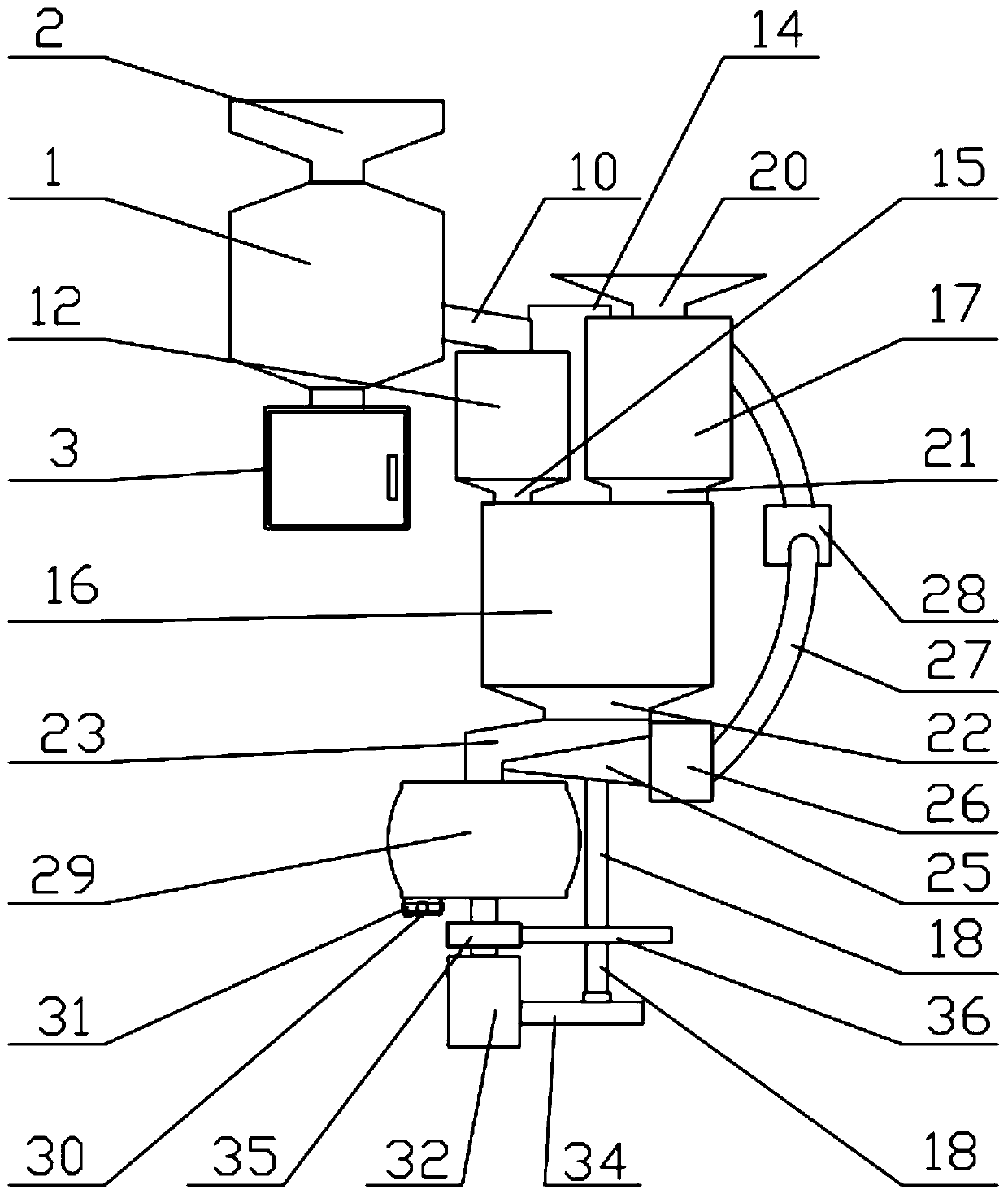
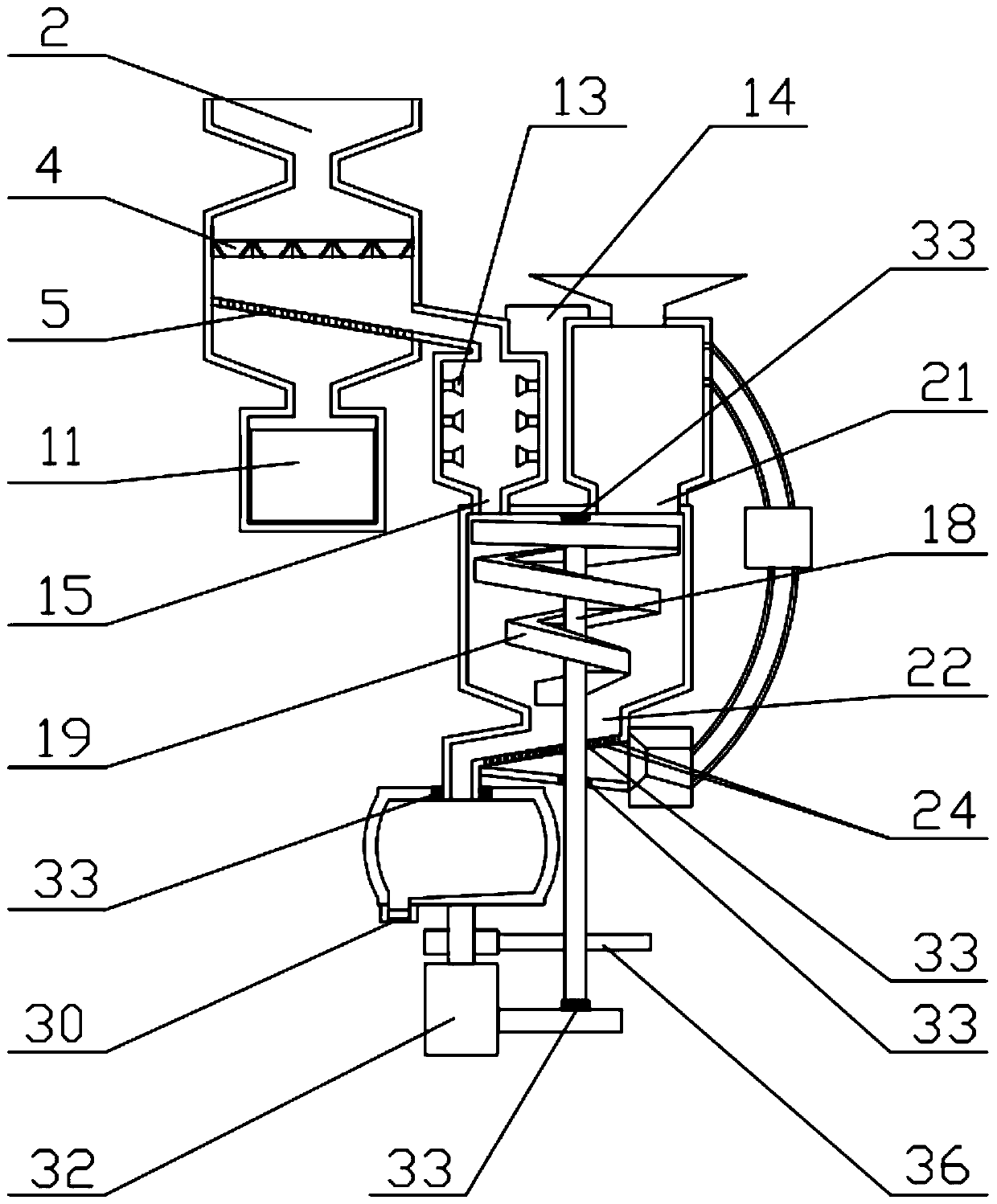
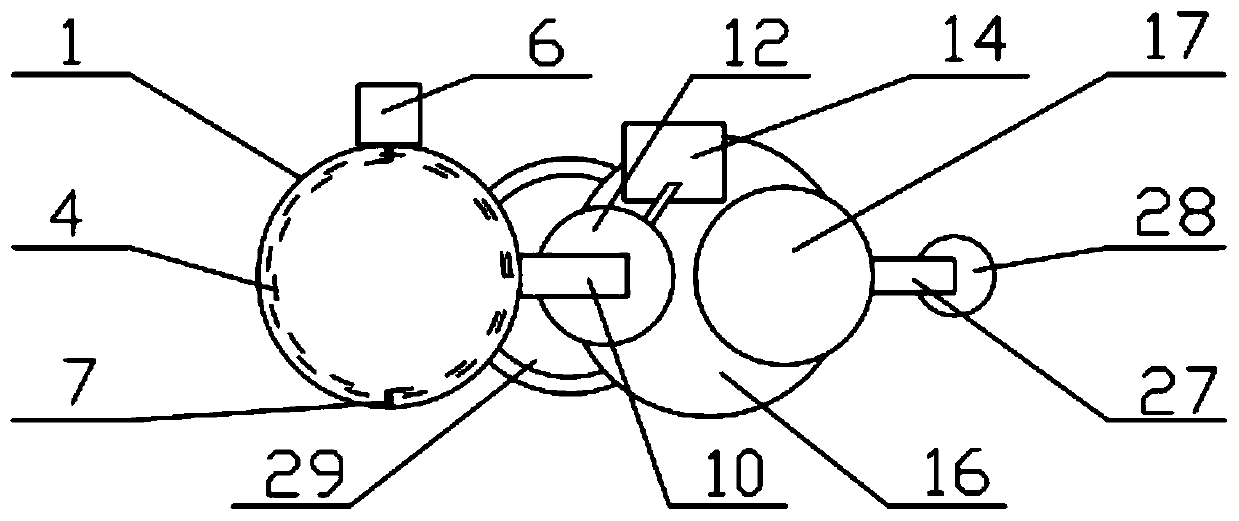
Image
Examples
Embodiment 1
[0055] A combination drug of repaglinide, comprising the following components in parts by mass:
[0056] Repaglinide 1 part;
[0057] 1 part of amobarbital;
[0058] 2 parts of water-soluble dietary fiber;
[0059] Glucose 0.2 parts;
[0060] 60 parts of filler;
[0061] 4 parts of disintegrant;
[0062] Adhesive 2 parts;
[0063] Arginine 2 parts.
[0064] specifically:
[0065] The water-soluble dietary fiber is lactoligosaccharide.
[0066] The filler is a composition of mannitol and microcrystalline cellulose, and the ratio of the mannitol to microcrystalline cellulose is 1:1 (including this ratio and the ratios described below are volume ratios).
[0067] The disintegrating agent is oligomeric hydroxypropyl cellulose.
[0068] The binder is a combination of povidone and hydroxypropyl methylcellulose, and the ratio of povidone and hydroxypropyl methylcellulose is 1:2.
[0069] The preparation method of the above-mentioned repaglinide combination medicine comprise...
Embodiment 2
[0076] A combination drug of repaglinide, comprising the following components in parts by mass:
[0077] Repaglinide 2 parts;
[0078] 1.5 parts of amobarbital;
[0079] 3 servings of water-soluble dietary fiber;
[0080] Glucose 0.3 parts;
[0081] 56 parts of filler;
[0082] 8 parts of disintegrant;
[0083] Adhesive 2 parts;
[0084] Arginine 1 part.
[0085] specifically:
[0086] The water-soluble dietary fiber is a mixture of xylooligosaccharide and hydroxymethylcellulose, and the ratio of xylooligosaccharide and hydroxymethylcellulose is 2:3.
[0087] The filler is mannitol.
[0088] The disintegrating agent is a composition of sodium hydroxymethyl starch and cross-linked sodium hydroxymethyl cellulose, and the ratio of sodium starch glycolate and sodium cross-linked hydroxymethyl cellulose is 1:1.
[0089] The binder is a combination of povidone, sodium hydroxymethylcellulose and hydroxypropylmethylcellulose, and their ratio is 1:1:2.
[0090] The preparatio...
Embodiment 3
[0097] A combination drug of repaglinide, comprising the following components in parts by mass:
[0098] Repaglinide 3 parts;
[0099] 2 parts of amobarbital;
[0100] 4 servings of water-soluble dietary fiber;
[0101] Glucose 0.4 parts;
[0102] 55 parts of filler;
[0103] 6 parts of disintegrant;
[0104] 3 parts adhesive;
[0105] Arginine 2 parts.
[0106] specifically:
[0107] The water-soluble dietary fiber is a mixture of lacto-oligosaccharides, xylo-oligosaccharides and pectin, and their ratio is 2:3:2.
[0108] The filler is a composition of microcrystalline cellulose and pregelatinized starch, and their ratio is 3:4.
[0109] The disintegrant is sodium hydroxymethyl starch.
[0110] The binder is hydroxypropyl methylcellulose.
[0111] The present invention also provides a preparation method of the repaglinide combination drug, which is characterized in that it comprises the following steps:
[0112] (I) Grinding and sieving: firstly crush and sieve each ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com