Preparation method of core-shell structure Te @ metal electrooxidation catalyst
A core-shell structure and catalyst technology, applied in the field of electrochemistry, can solve the problems of large difference in reduction potential, difficulty of PtRu alloy, high cost of platinum and palladium, and achieve the improvement of electron transport rate, good methanol oxidation performance, and improved catalytic activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
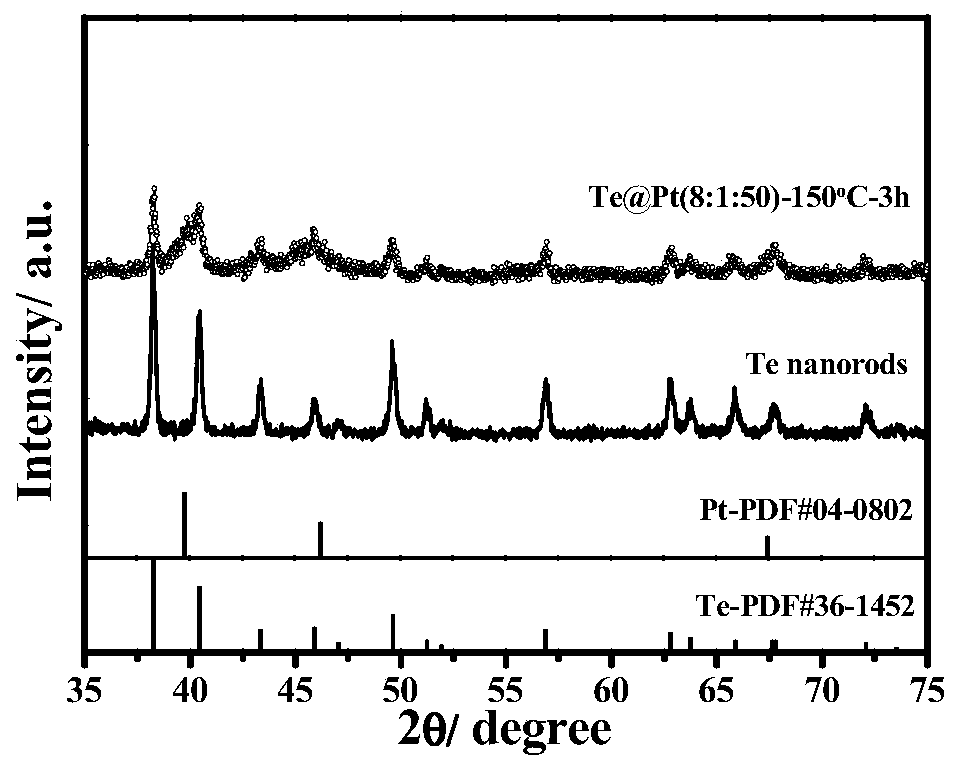
[0053] Preparation of core-shell structure Te@Pt(8:1:50)-150℃-3h electrooxidation catalyst:
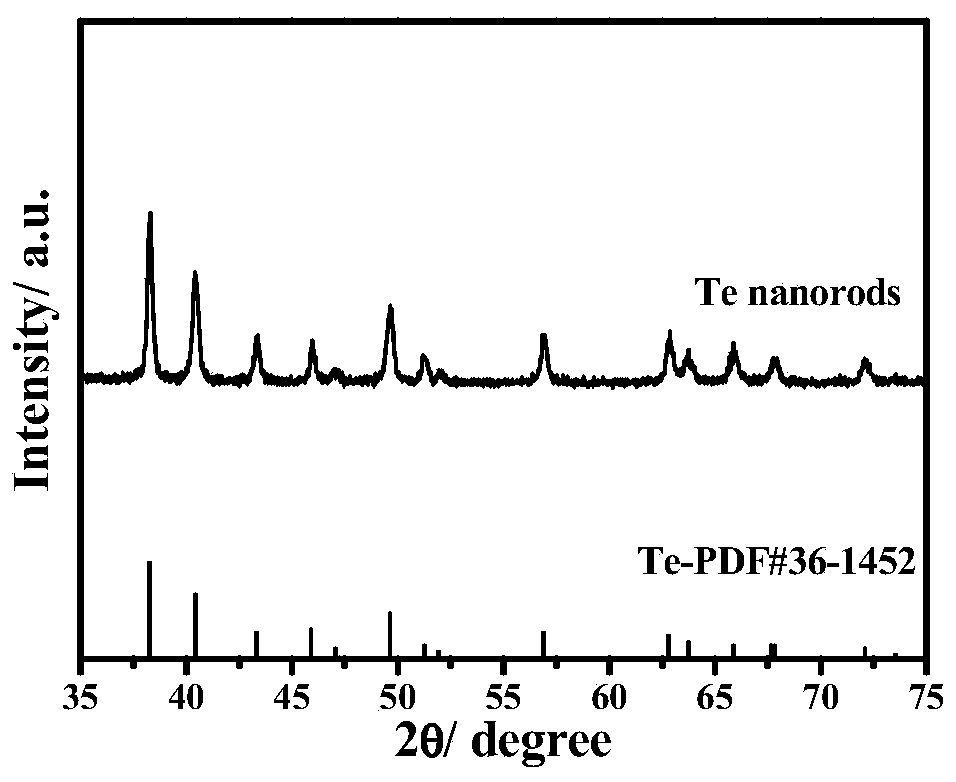
[0054] In the first step, 230 mg of sodium tellurite, 450 mg of ascorbic acid, and 100 mg of polyvinylpyrrolidone were sequentially added to a 100 mL flat-bottomed flask containing 30 mL of ethylene glycol, and stirred at room temperature until uniformly mixed. The above solution was transferred to a Teflon substrate with a volume of 100 mL, and the temperature of the oven was set at 150° C., and the holding time was 6 h. After the reaction was completed and cooled to room temperature, it was washed with deionized water, acetone, and ethanol several times, filtered by suction, and vacuum-dried to obtain a silvery white glossy substance, which was tested by a powder XRD diffractometer and the sample was Te, which was used for the next experiment.
[0055] The second step, at room temperature, sequentially 16mgTe, 66uLH 2 PtCl 6 The solution (Pt: 30 mg / mL) and 100 mg of polyvinylpyrro...
Embodiment 2
[0063] Preparation of core-shell structure Te@Pt(8:1:300)-150℃-3h electro-oxidation catalyst: at room temperature, take Te 16mg prepared in Example 1, 66uLH 2 PtCl 6 The solution (Pt: 30mg / mL) and 600mg of polyvinylpyrrolidone were added to a 250mL flat-bottomed flask containing 100mL of ethylene glycol, and magnetically stirred until uniformly mixed. The reaction temperature was adjusted to 150° C. by means of oil bath heating, and the holding time was 3 hours. Cool to room temperature, wash and dry to obtain a core-shell structure Te@Pt (8:1:300)-150°C-3h electro-oxidation catalyst.
[0064] Figure 7 The XRD pattern of the core-shell structure Te@Pt(8:1:300)-150°C-3h electrooxidation catalyst prepared in Example 2, it can be seen from the figure that the main characteristic peaks of Te still exist, and there are also Pt peaks. Three characteristic peaks at 39.7°, 46.2° and 67.4° correspond to the (111), (200) and (220) crystal planes of Pt, respectively, indicating the f...
Embodiment 3
[0068] Preparation of core-shell structure Te@Pt(8:1:50)-150℃-6h electrooxidation catalyst: at room temperature, sequentially take Te16mg prepared in the first step of Example 1, 66uLH 2 PtCl 6 The solution (Pt: 30mg / mL) and 100mg of polyvinylpyrrolidone were added to a 250mL flat-bottomed flask containing 100mL of ethylene glycol, and magnetically stirred until uniformly mixed. Using oil bath heating method, adjust the reaction temperature to 150°C, and the holding time is 6h. Cool to room temperature, wash and dry to obtain a core-shell structure Te@Pt (8:1:50)-150°C-6h electro-oxidation catalyst.
[0069] Figure 10 The XRD pattern of the core-shell structure Te@Pt(8:1:50)-150°C-6h electro-oxidation catalyst prepared in Example 3, it can be seen from the figure that the catalyst has three obvious Pt characteristic peaks , corresponding to the (111), (200) and (260) crystal planes of Pt at 39.7°, 46.2° and 67.4°, respectively, indicating the formation of Pt.
[0070] F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com