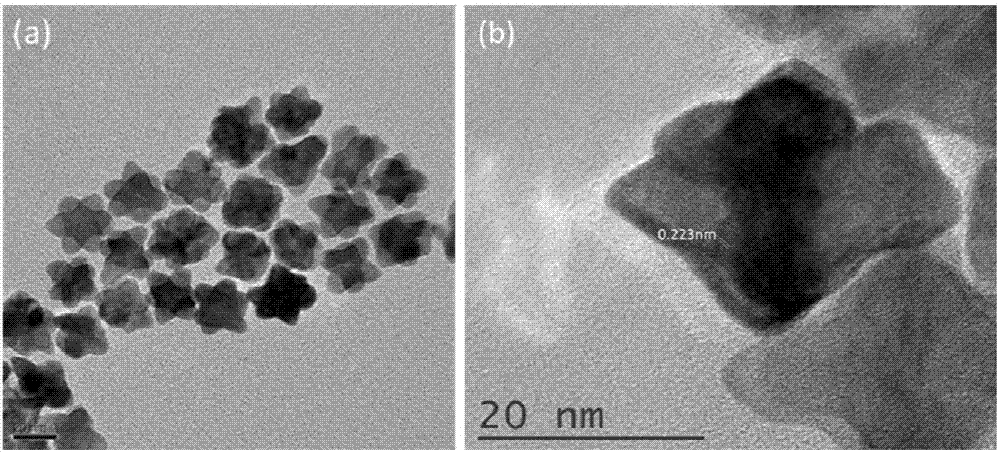
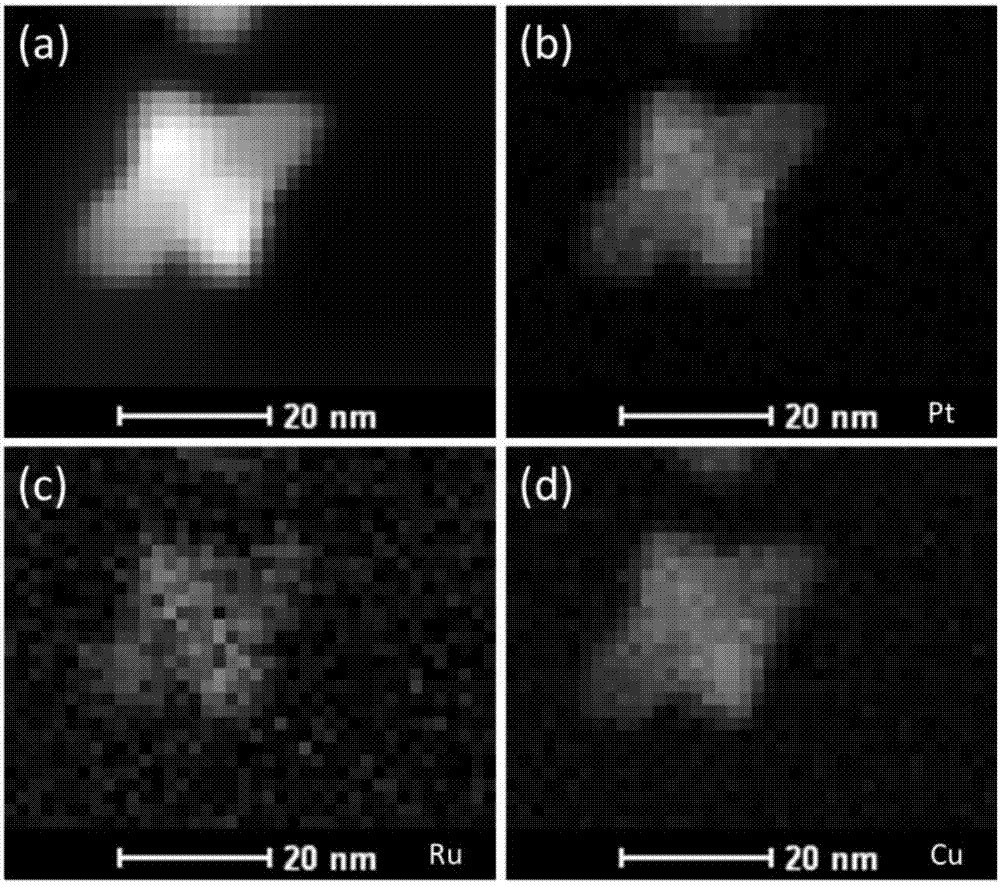
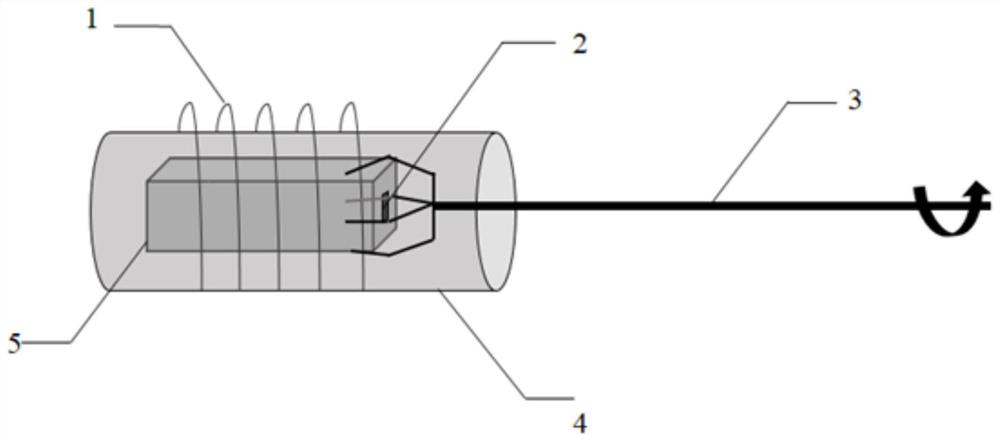
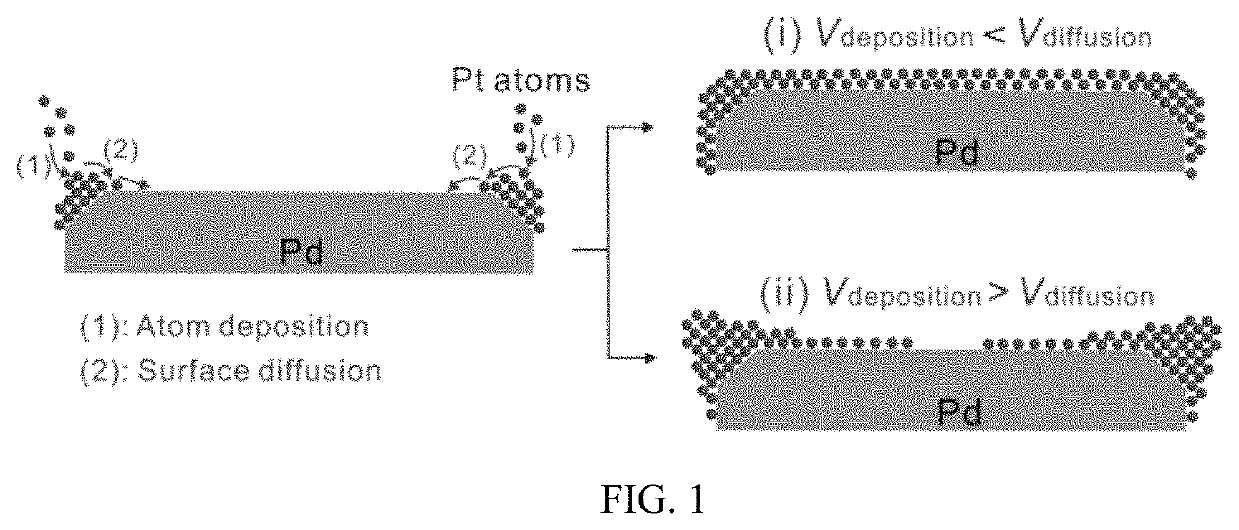
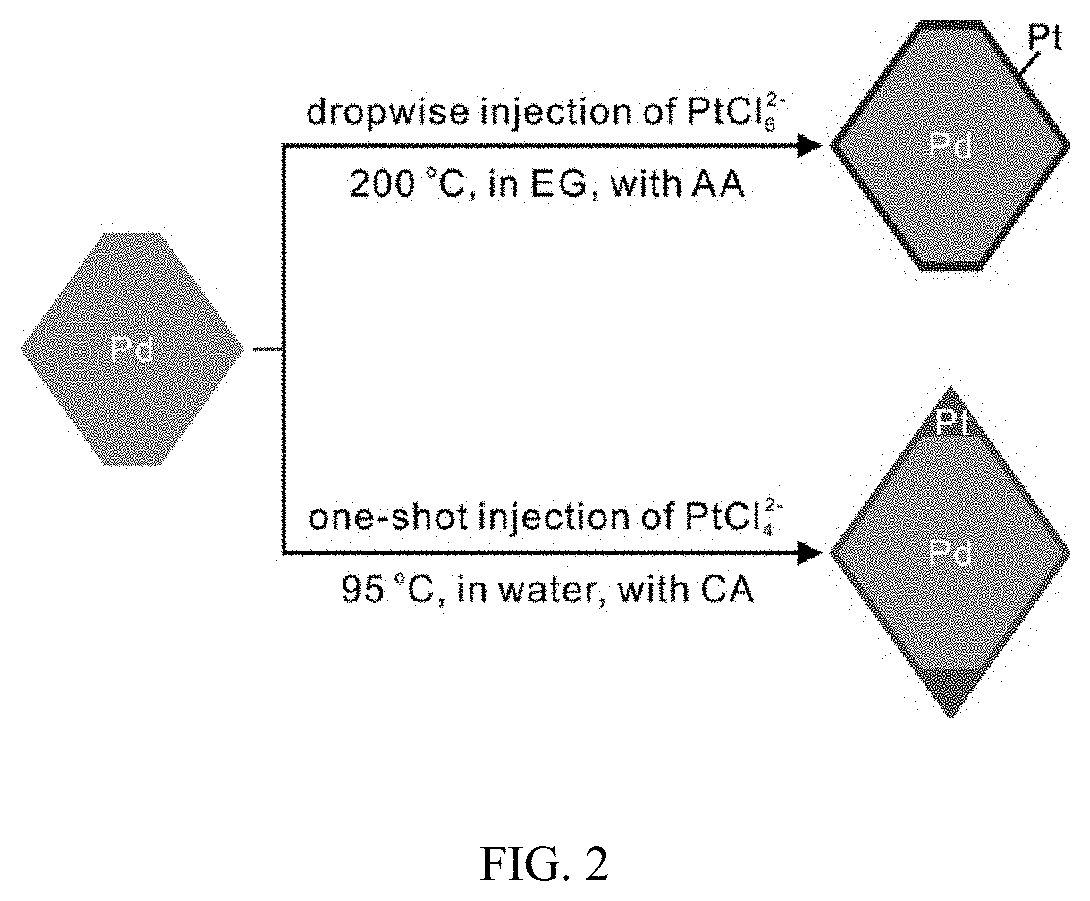
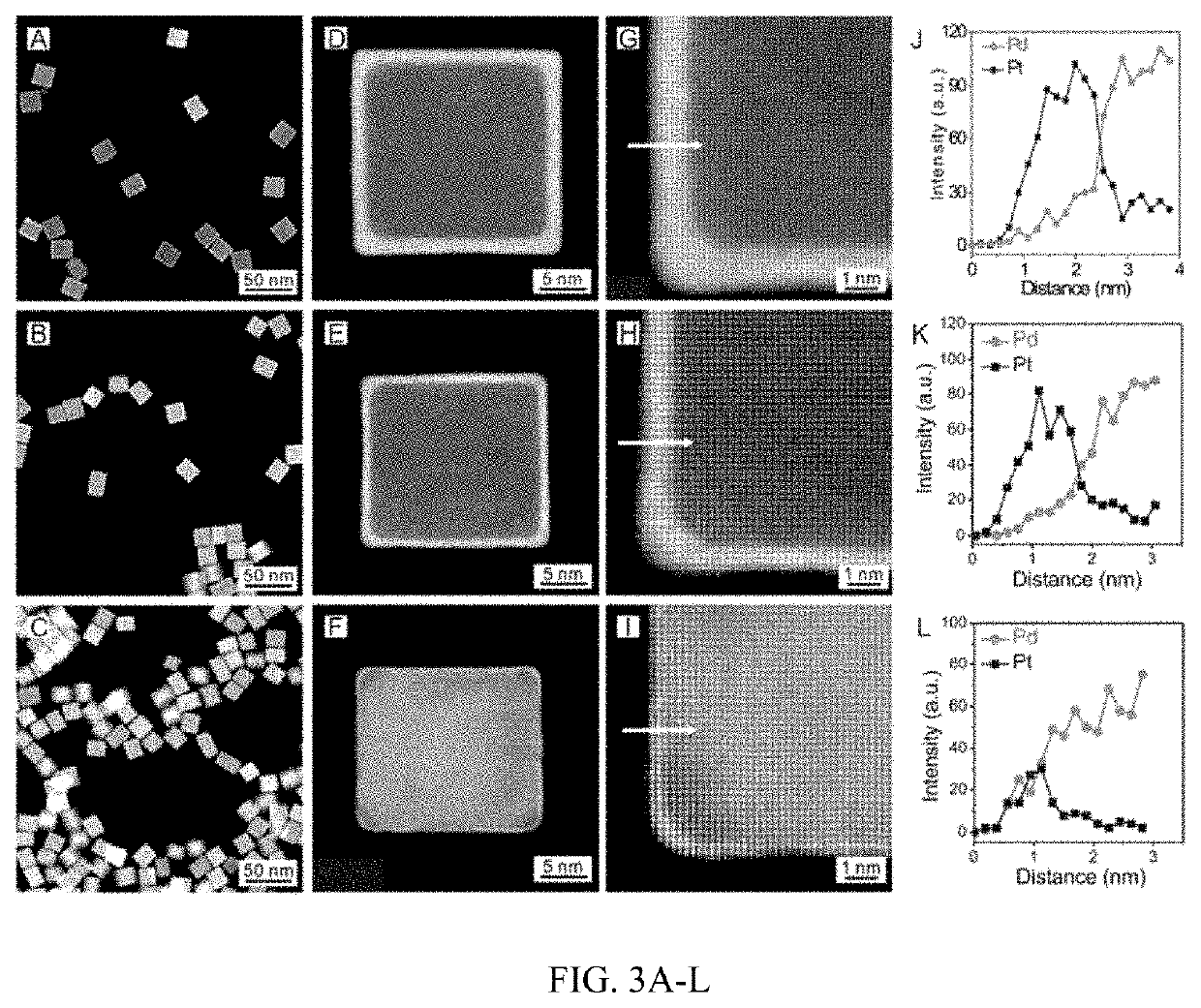
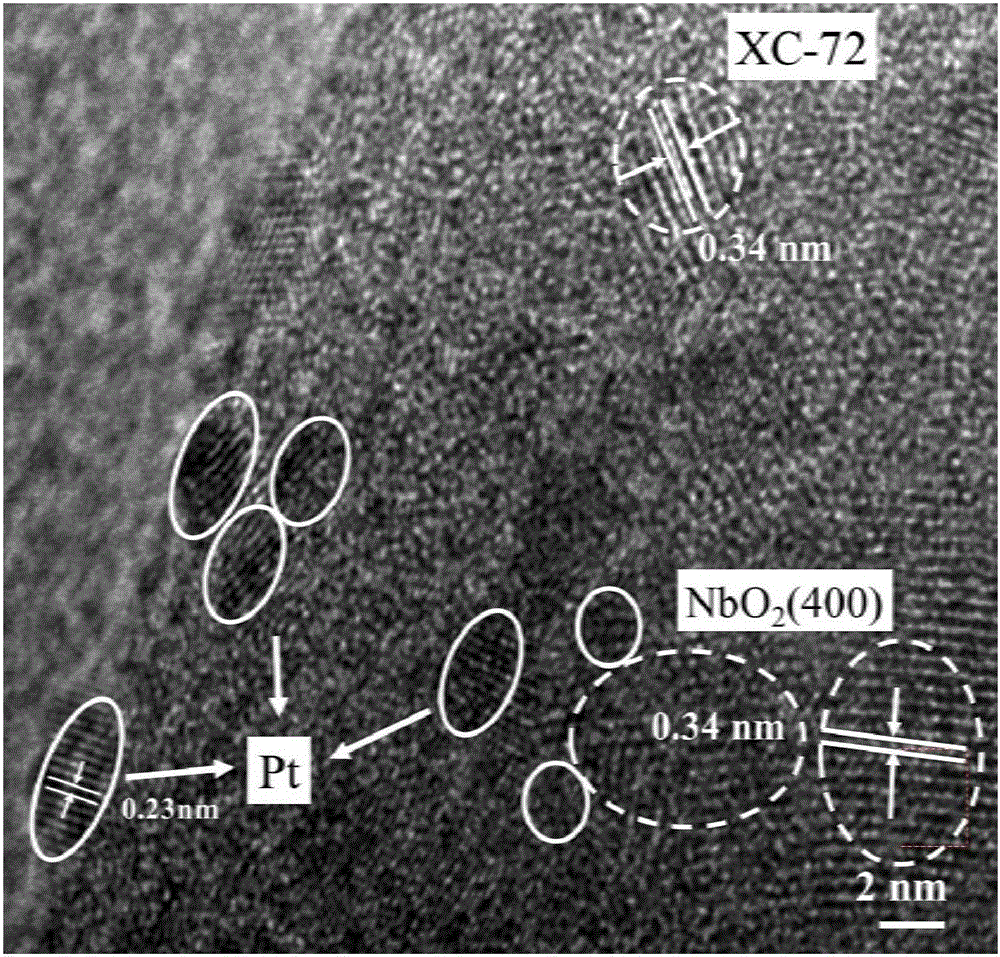
Patents
Literature
56 results about "Mass activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
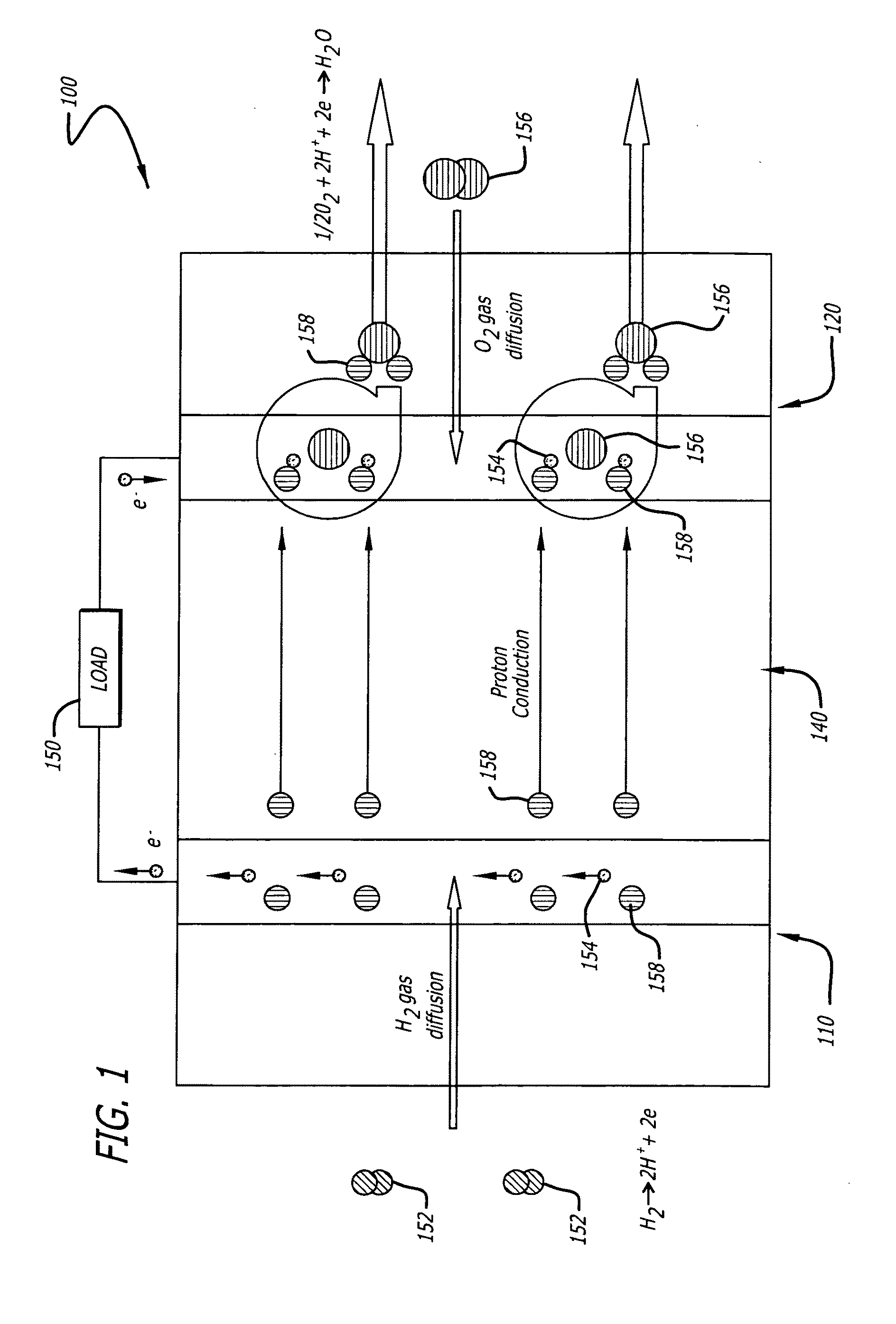
Platinum and Platinum Based Alloy Nanotubes as Electrocatalysts for Fuel Cells
ActiveUS20090220835A1Easy to chargeEnhances mass transportationMaterial nanotechnologyPhysical/chemical process catalystsDegradation pathwayAlloy
Electrocatalyst durability has been recently recognized as one of the most important issues that have to be addressed before the commercialization of the proton exchange membrane fuel cells (PEMFCs). The present invention is directed to a new class of cathode catalysts based on supportless platinum nanotubes (PtNTs) and platinum alloy nanotubes, for example, platinum-palladium nanotubes (PtPdNTs), that have remarkable durability and high catalytic activity. Due to their unique combination of dimensions at multiple length scales, the platinum nanotubes of the present invention can provide high platinum surface area due to their nanometer-sized wall thickness, and have the potential to eliminate or alleviate most of the degradation pathways of the commercial carbon supported platinum catalyst (Pt / C) and unsupported platinum-black (PtB) as a result of their micrometer-sized length. The platinum nanotube catalysts of the present invention asymptotically approach a maximum of about twenty percent platinum surface area loss in durability test, while the commercial PtB and Pt / C catalysts lose about fifty-one percent and ninety percent of their initial surface area, respectively. Moreover, the PtNT and PtPdNT catalysts of the present invention show higher mass activity and much higher specific activity than commercial Pt / C and PtB catalysts.
Owner:RGT UNIV OF CALIFORNIA
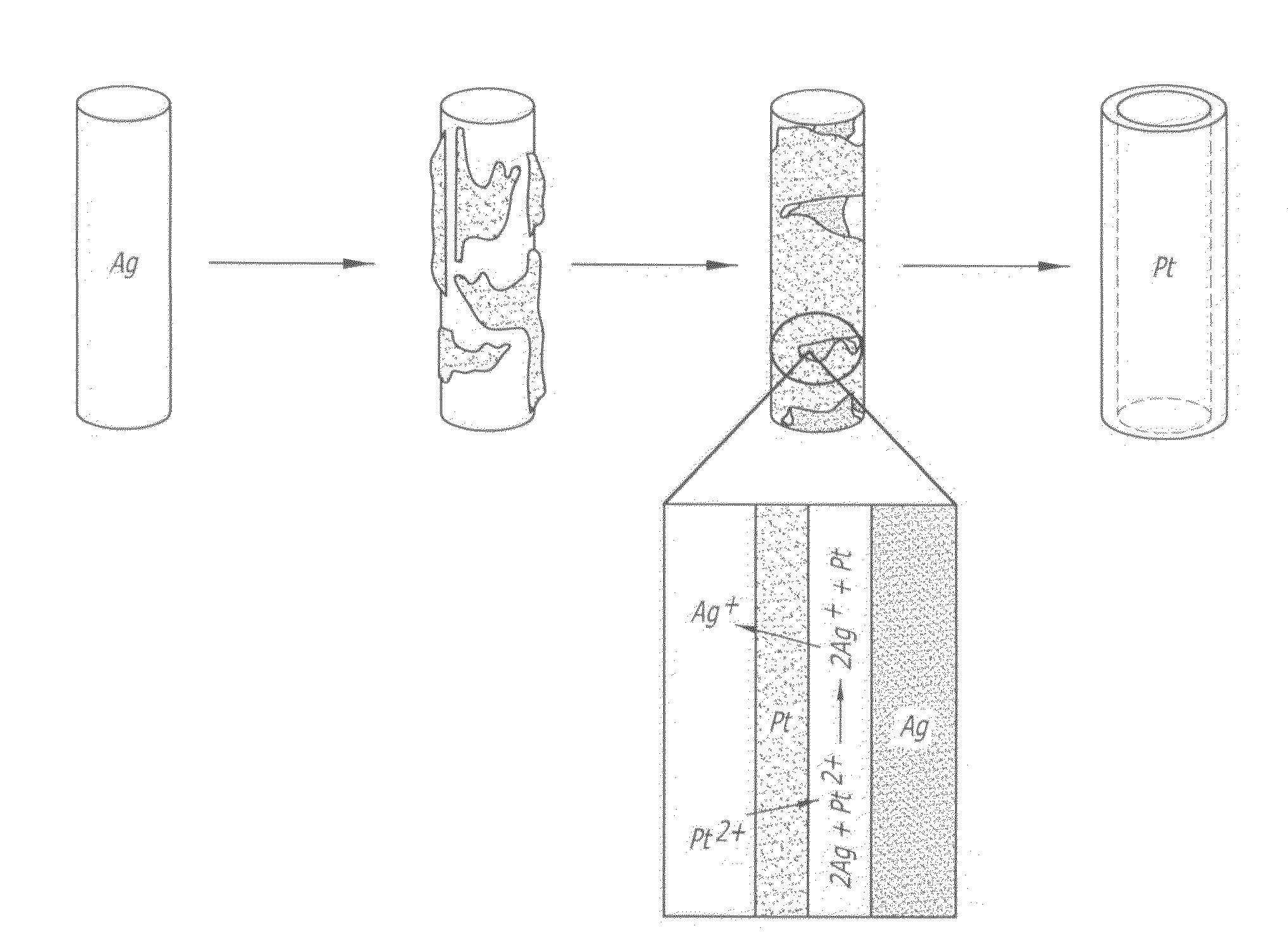
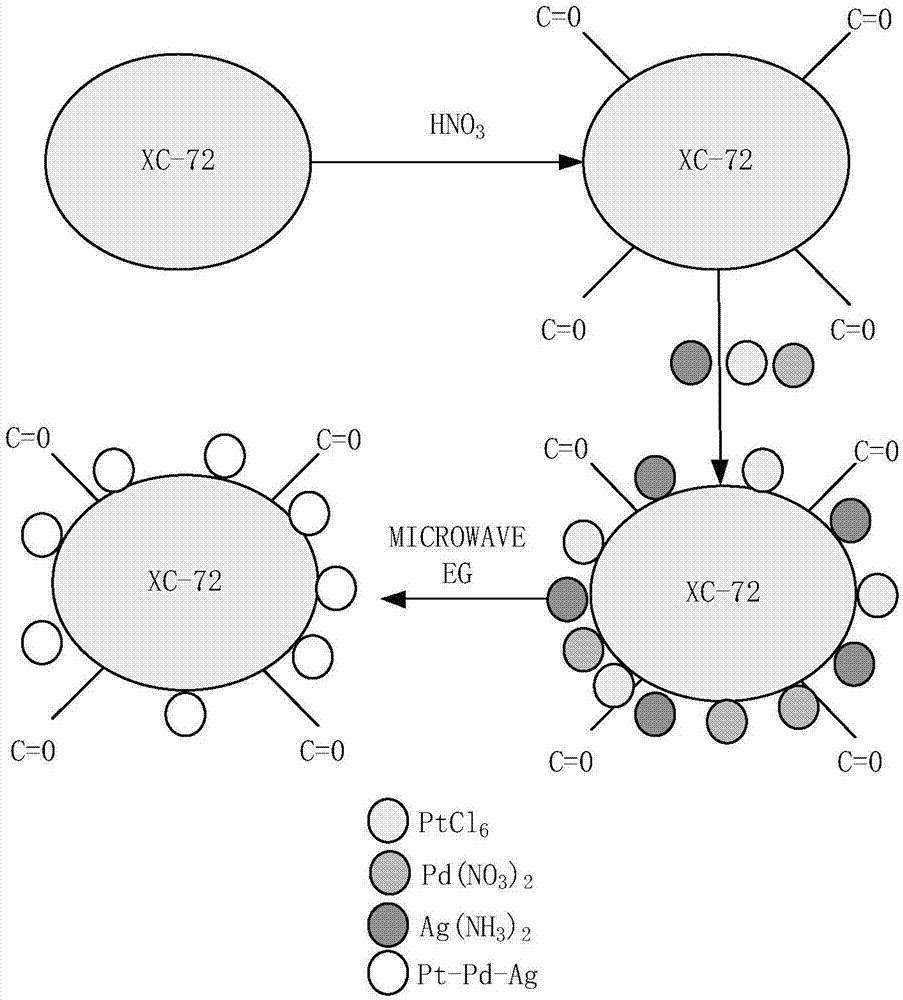
Carbon-based platinum-silver-palladium ternary alloy catalyst and preparation method thereof
ActiveCN107887618AHigh activityImprove stabilityMaterial nanotechnologyCell electrodesPlatinumSolvent
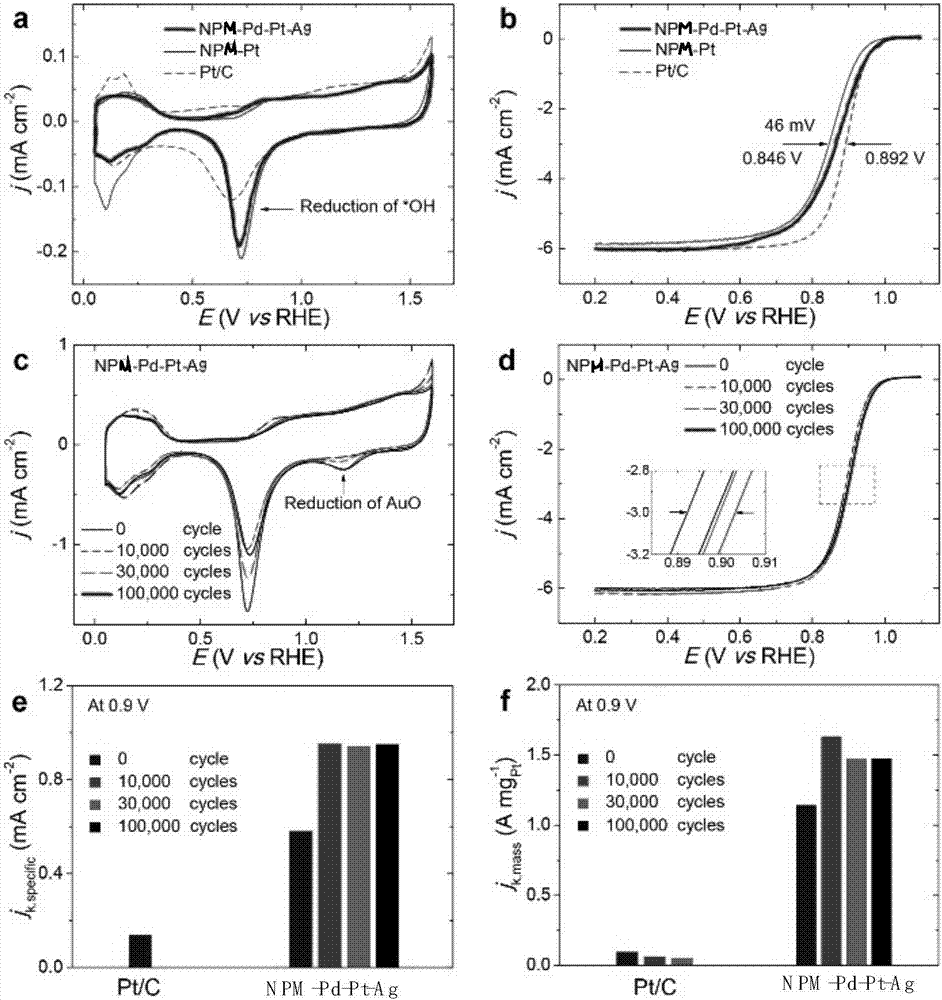
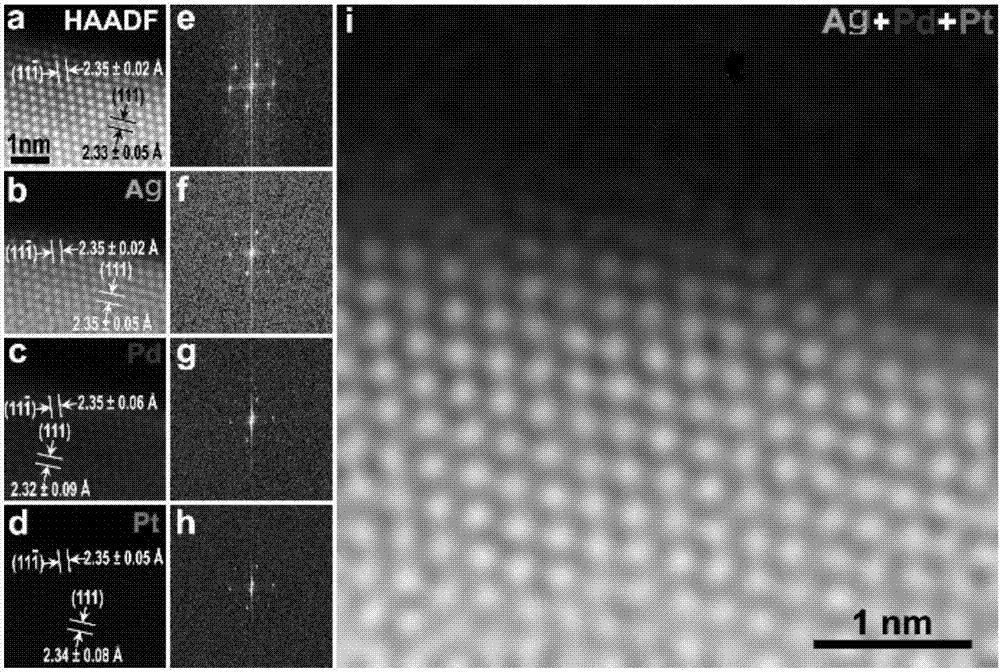
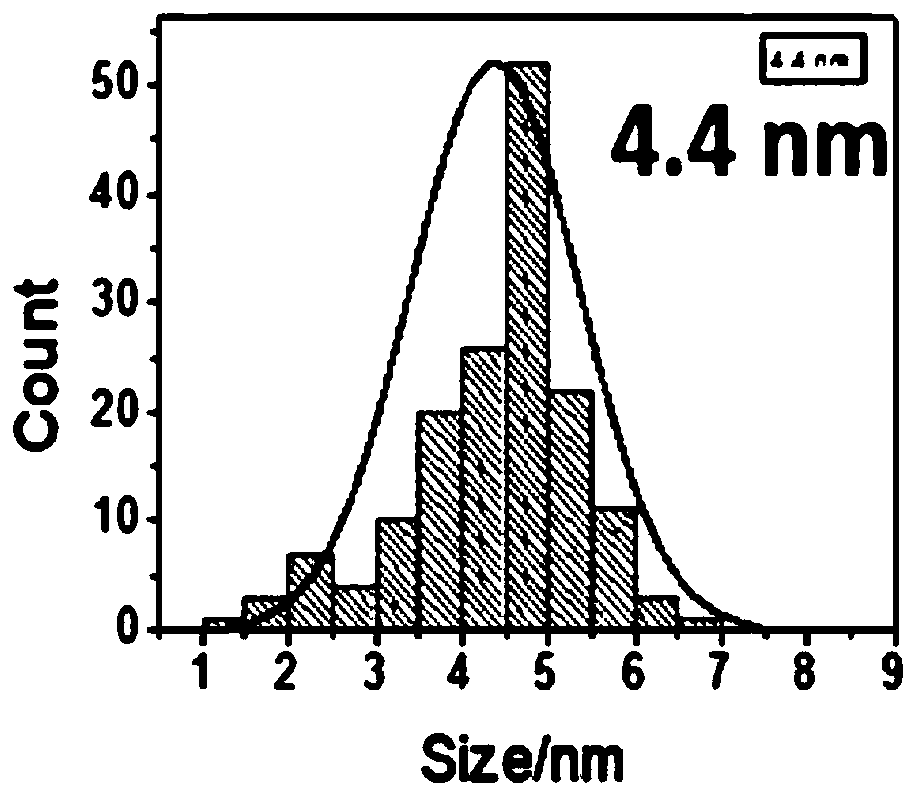
The invention provides a carbon-based platinum-silver-palladium ternary alloy catalyst. According to the catalyst, a platinum-silver-palladium ternary alloy is uniformly supported on a carbon black surface with a hollow structure; the platinum-silver-palladium ternary alloy is an alloy solid solution taking silver as a solvent and taking platinum and palladium as solutes, and the particle size ofthe supported alloy particles is 6-8nm. The invention further provides preparation and application of the carbon-based platinum-silver-palladium ternary alloy catalyst. The carbon-based platinum-silver-palladium ternary alloy catalyst provided by the invention is a platinum-palladium-silver (Pt-Pd-Ag) ternary alloy layer redox catalyst with low Pt capacity, and an oxygen reduction catalyst with high activity and high stability. The mass activity of the catalyst is about 12 times that of a commercial Pt / C catalyst, and after tested by 50000 cycles, the stability of the catalyst is also kept invariable.
Owner:江苏群菱能源科技有限公司
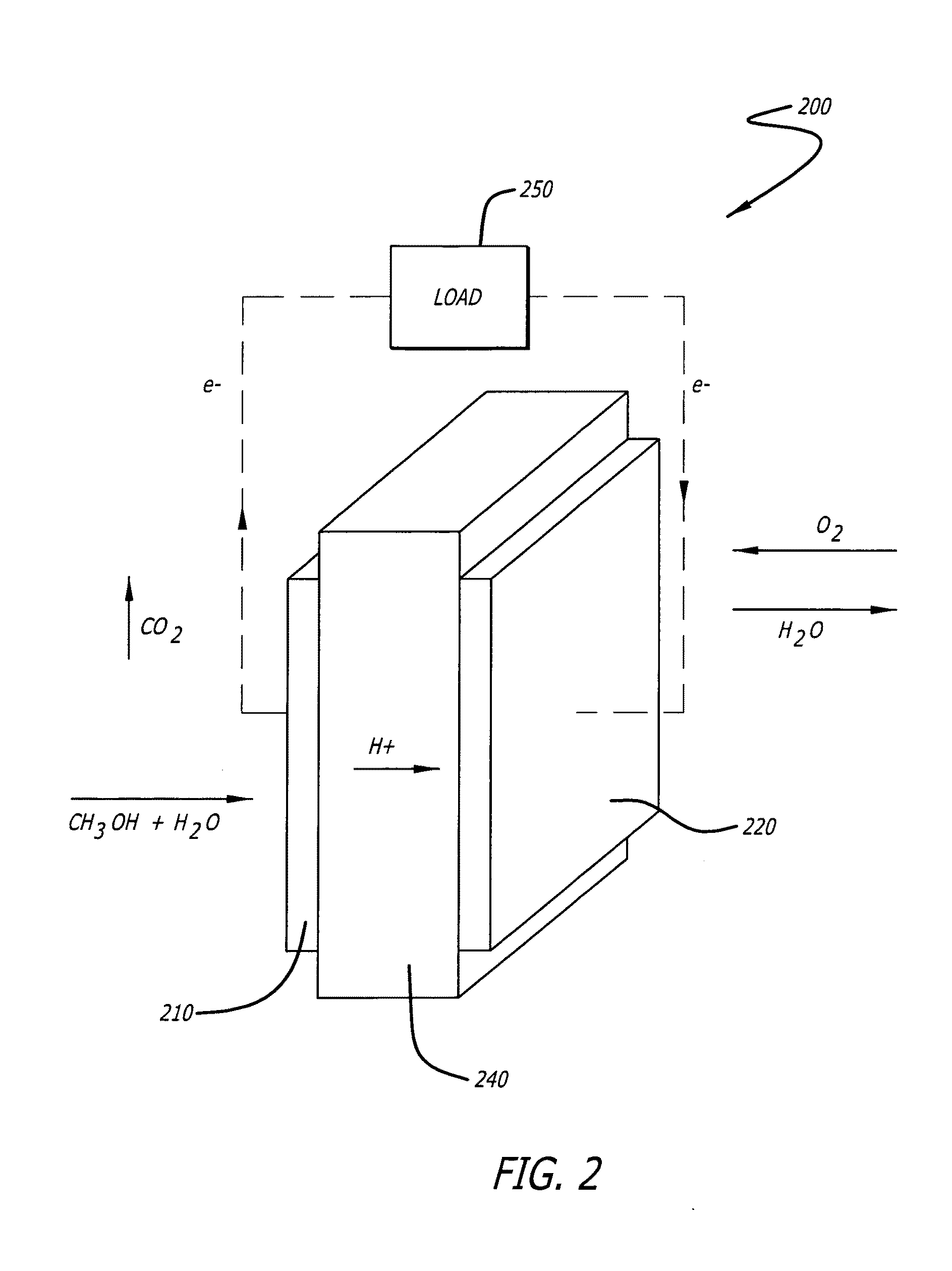
Fuel Cells with Sputter Deposited Pt and Pt Alloy Electrodes
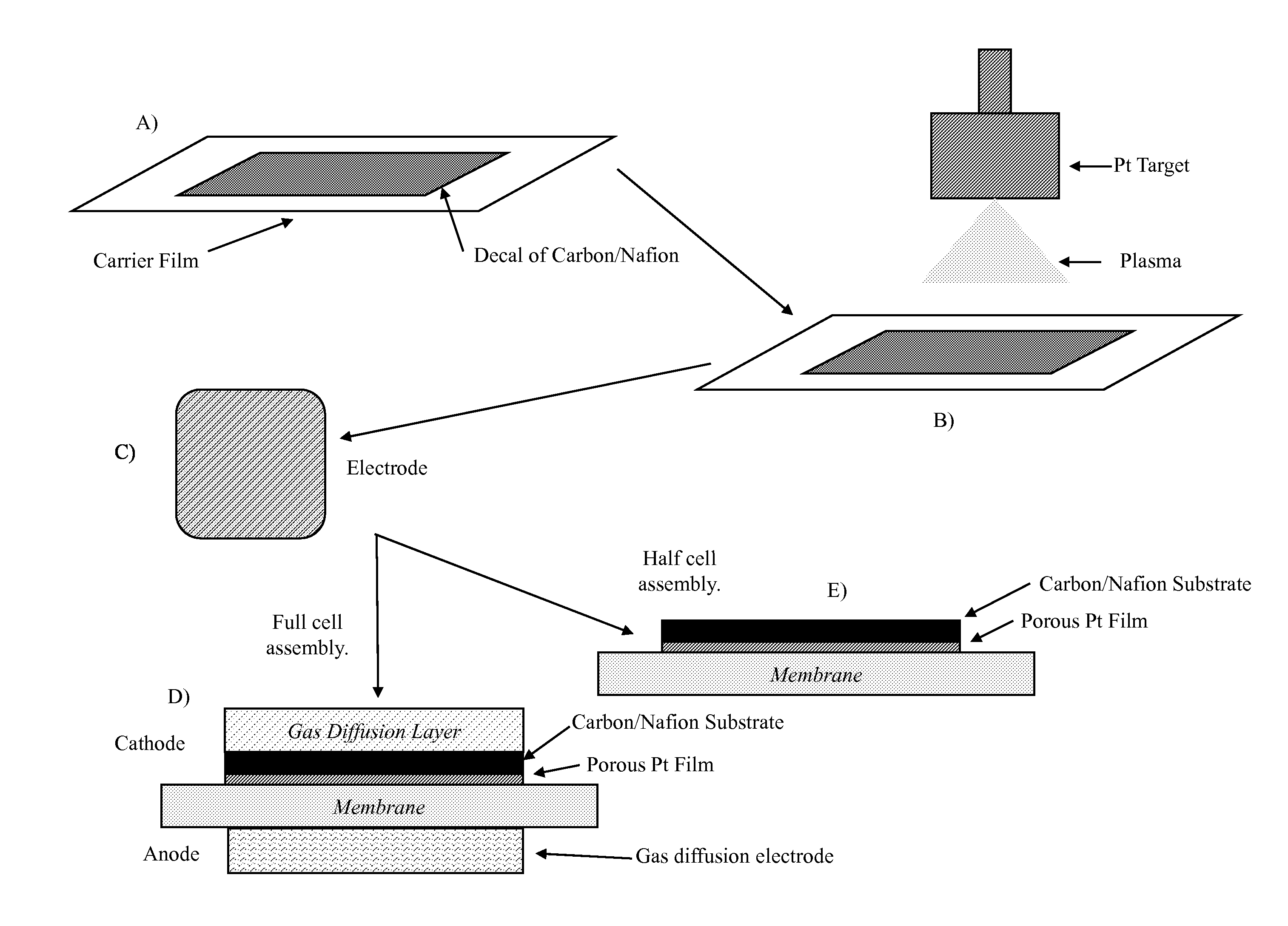
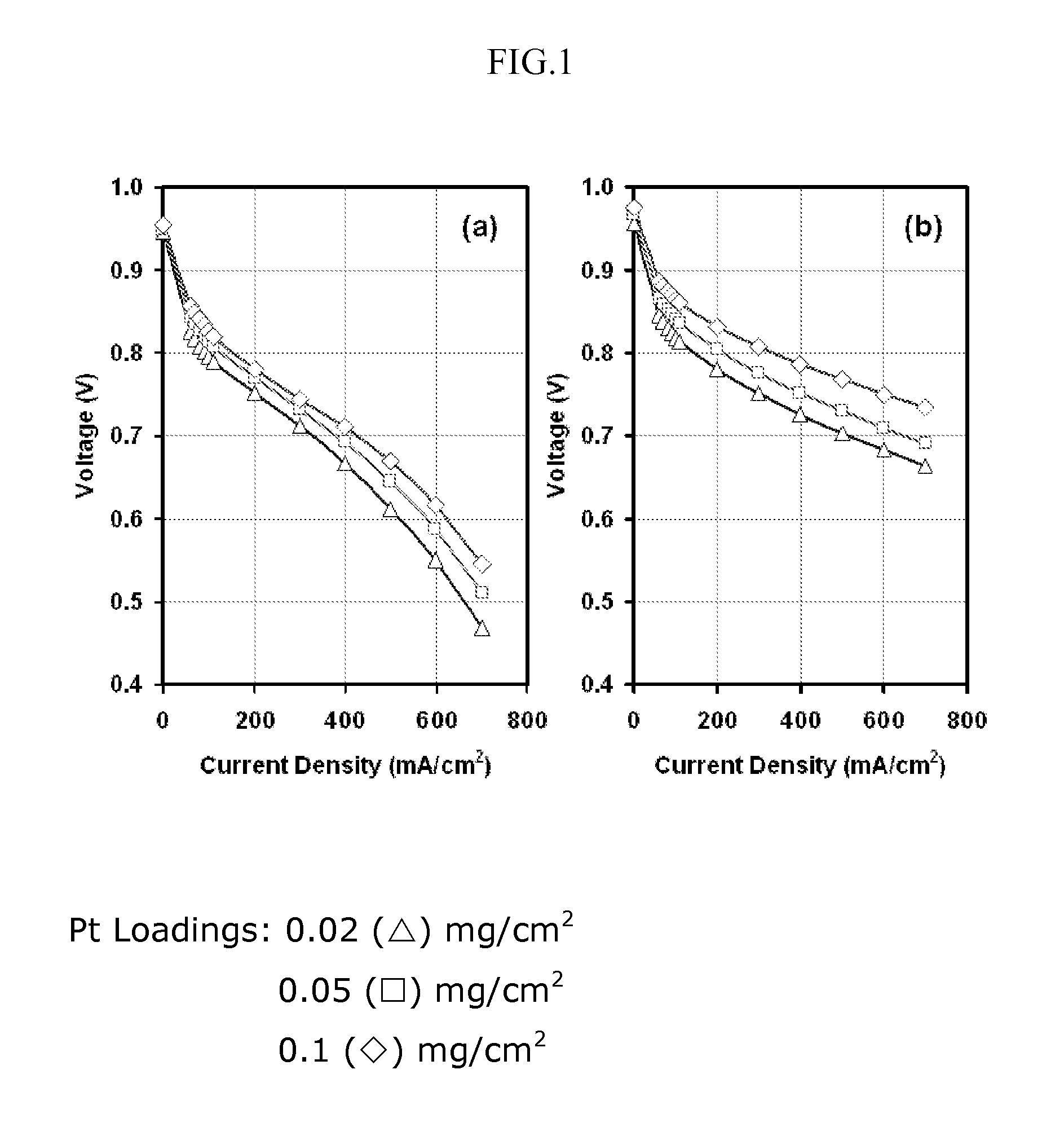
The present application is directed to a fabrication method to reduce Pt loading in fuel cells through the use of thin film electrodes by increasing Pt utilization and the use of more active Pt alloys that can be easily and inexpensively fabricated by sputter deposition. Pt and Pt alloy thin films were sputter deposited onto carbon / Nafion® decals and subsequently hot pressed with the catalyst thin film towards the membrane. The results show improved mass performance and catalyst utilization with Pt thin films and increased mass activities can be achieved with PtCo (76:24 atomic ratio) and PtCr (80:20 atomic ratio) as compared to pure Pt. Mass activity improvements of 14 mV and 8 mV were observed for the PtCo and PtCr alloys with respect to a pure Pt film with similar mass loading under 300 / 350 kPa hydrogen / oxygen operation.
Owner:HONDA MOTOR CO LTD
Platinum-palladium catalyst with intermediate layer
InactiveUS20120329642A1Increased mass activityHigh activityCell electrodesMetal/metal-oxides/metal-hydroxide catalystsPlatinumFuel cells
A fuel cell catalyst comprises a support having a core arranged on the support. In one example, the core includes palladium nanoparticles. A layer, which is gold in one example, is arranged on the core. A platinum overlayer is arranged on the gold layer. The intermediate gold layer greatly increases the mass activity of the platinum compared to catalysts in which platinum is deposited directly onto the palladium without any intermediate gold layer.
Owner:AUDI AG
PtRu/C catalyst and its preparation method
InactiveCN104209122AHigh activityImprove stabilityCell electrodesMetal/metal-oxides/metal-hydroxide catalystsRoom temperatureSodium borohydride
The invention provides a preparation method of a PtRu / C catalyst, and belongs to the field of catalyst preparation methods. The method comprises the following steps: dispersing a carbon carrier in water to obtain a first suspension; adding a chloroplatinic acid solution and a ruthenium chloride solution to the first suspension to obtain a second suspension; and pouring the second suspension into a sodium borohydride solution, and stirring at room temperature to obtain the PtRu / C catalyst. The invention also provides the PtRu / C catalyst. The PtRu / C catalyst has the characteristics of small particle size, uniform dispersion, and good methanol electrooxidation reaction catalysis performance. Experiments show that when the capacity of the PtRu / C catalyst prepared through the method is 30wt%, the mass activity of the catalyst in a methanol solution with sulfuric acid as an electrolyte reaches 447mA / mg, and is 1 time higher than the mass activity of commercial PtRu / C catalysts.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
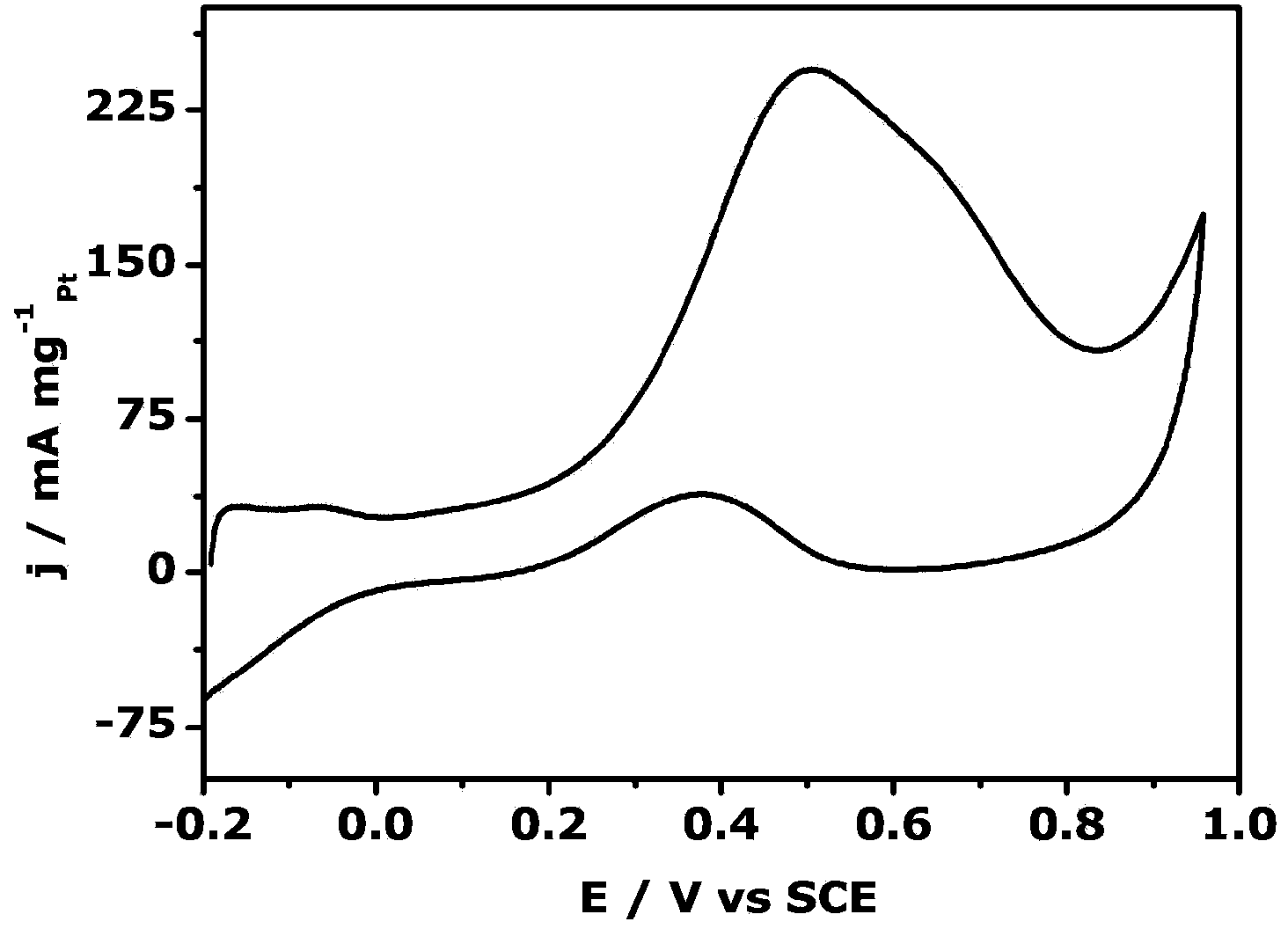
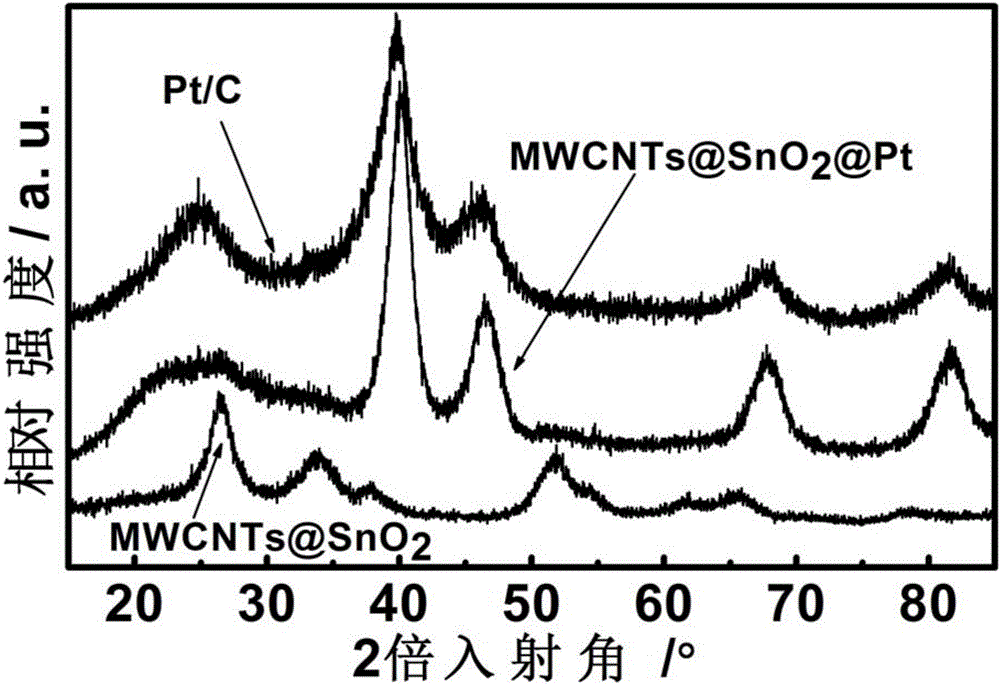
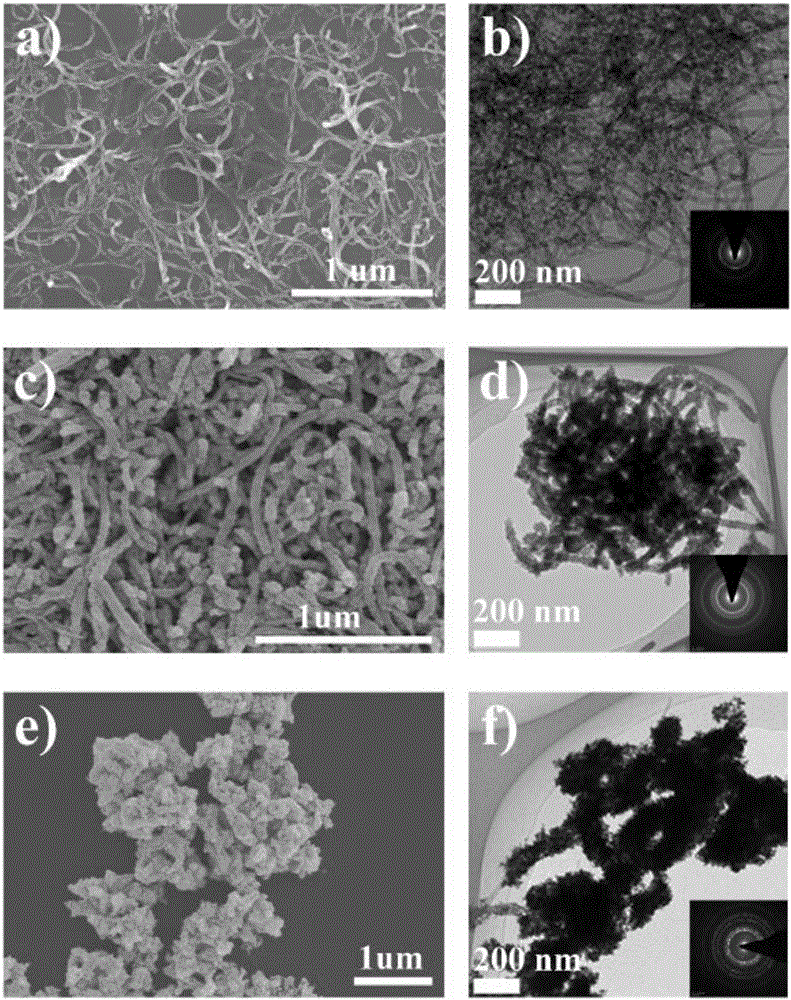
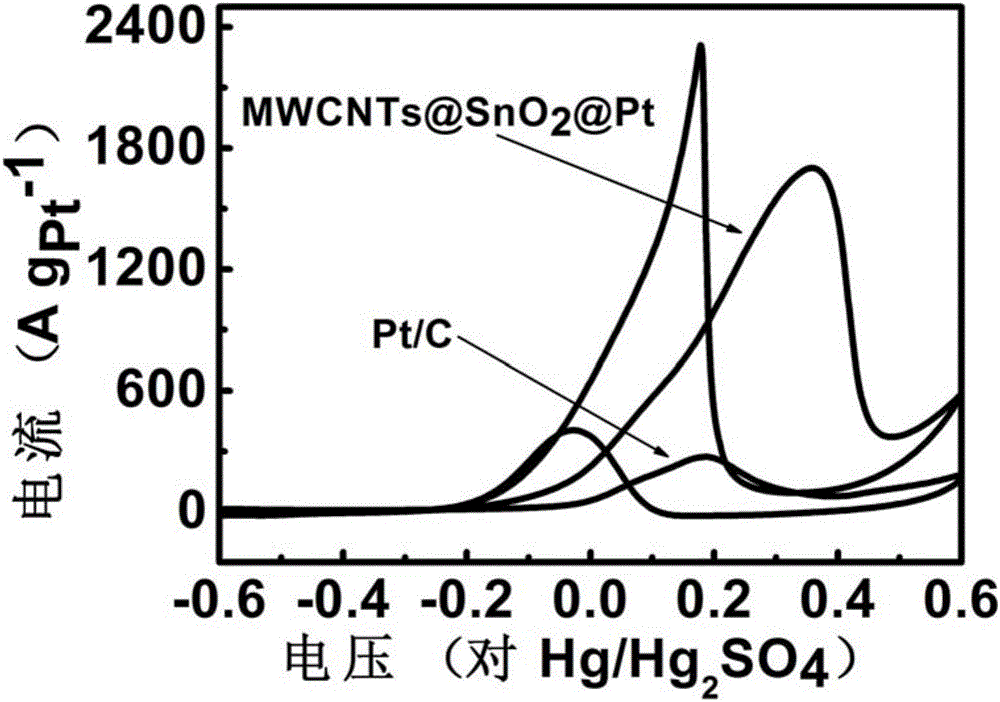
Platinum-based nanoparticle coating and tin dioxide covering carbon nanotube and preparation method thereof
ActiveCN106784900AImprove the quality of catalytic activityImprove catalytic stabilityCell electrodesTin dioxideCatalytic oxidation
The invention relates to a platinum-based nanoparticle coating and tin dioxide covering carbon nanotube and a preparation method thereof, wherein the carbon nanometer tube is a multi-wall carbon nanotube; a layer of tin dioxide is loaded on the carbon nanotube; a layer of platinum-based metal is loaded on the tin dioxide layer; the platinum-based metal exists in a nanometer particle form, and is connected into a network structure. The catalyst provided by the invention has the advantages that the noble metal utilization rate is high; the catalytic activity and the carbon monoxide poisoning resistance performance of the catalyst are high; the service life of the catalyst is long; the methanol catalytic oxidation activity is more than 6.2 times of the commercial Pt / C catalyst; the oxygen reduction mass activity at the hydrogen scale potential being 0.9V is 9.6 times of that of the commercial Pt / C catalyst; the granularity is preferably between 1.0 to 10.0nm, and is also preferably between 2.0 to 4.0nm.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Embedded alloy catalyst and preparation method and application thereof
InactiveCN111146446AHigh reactivityImprove electronic conductivityCell electrodesSolid electrolyte fuel cellsPtru catalystMetallurgy
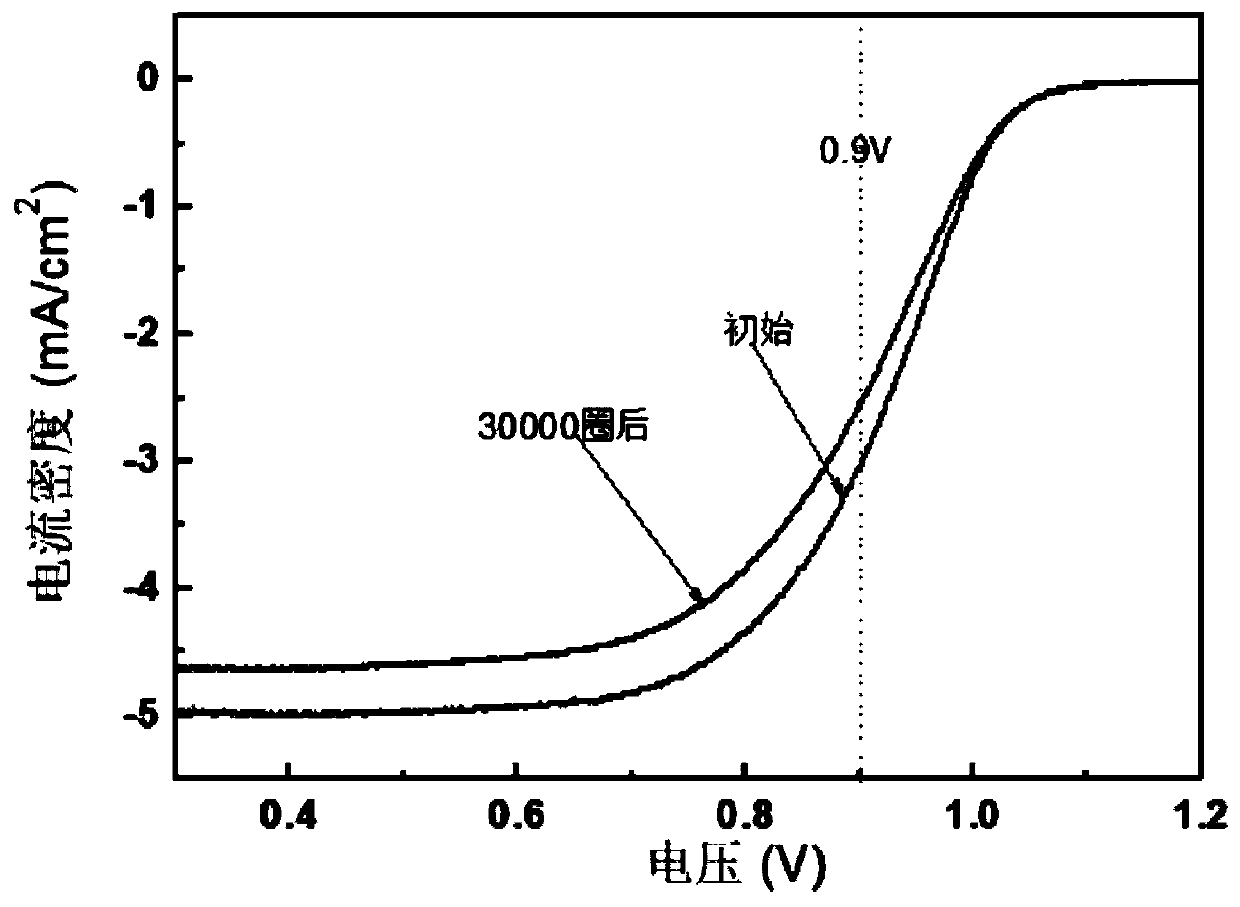
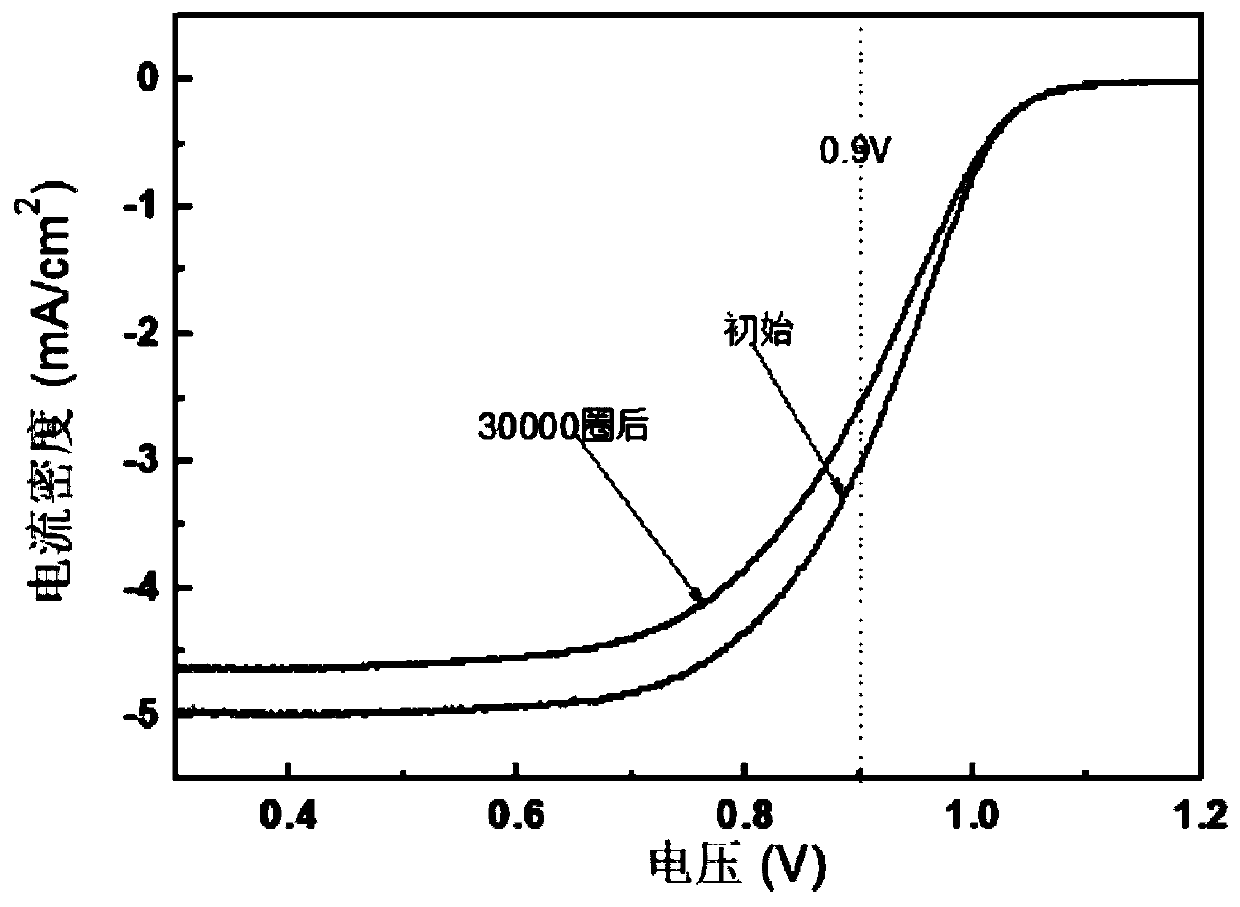
The invention relates to an embedded alloy catalyst and a preparation method and application thereof. The alloy catalyst comprises a carbon substrate material, a nitrogen-doped carbon material locatedon the surface of the carbon substrate material and noble metal nanoparticles, and the noble metal nanoparticles are embedded into the nitrogen-doped carbon material; the electron conduction capability and the oxygen reduction activity of the alloy catalyst are improved through the synergistic effect between the nitrogen-doped carbon material and the noble metal nanoparticles in the alloy catalyst; if the noble metal is Pt, the mass activity of the alloy catalyst can be more than two times that of traditional platinum carbon, the alloy catalyst has excellent stability, and the activity attenuation is 31% or below after 30,000 circles of accelerated circulation; and a membrane electrode of 5*10cm2 is prepared from the graphene oxide, a single battery is assembled for testing, and it is ensured that the current density can reach 2800mA / cm2 at 0.6V under the conditions that the battery temperature is 75 DEG C, the humidifying temperature is 70 DEG C, the RH is 60% and the stoichiometricratio of hydrogen to air is 1.2 / 2.2.
Owner:FAW JIEFANG AUTOMOTIVE CO
Fuel cell electrode catalyst and manufacturing method thereof, supporting electrode of fuel cell electrode catalyst, and fuel cell
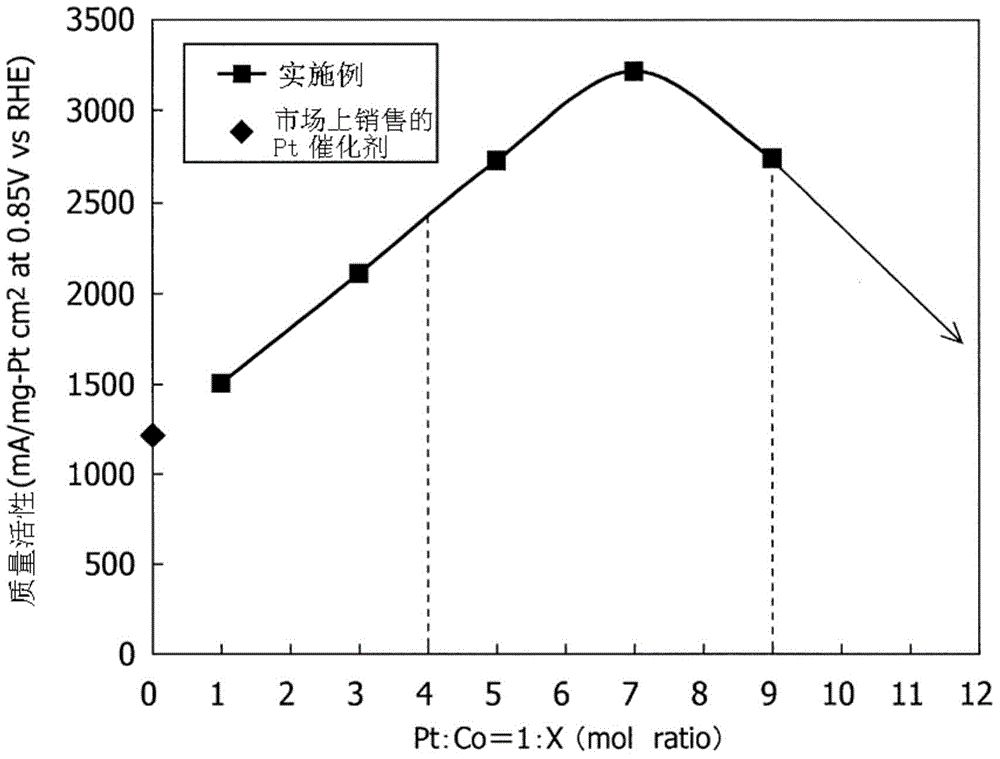
The invention provides a fuel cell electrode catalyst capable of greatly reducing the use of Pt in PtCo alloy and obtaining PtCo / C which can achieve a mass activity equal to that of a Pt / C catalyst or above, and a manufacturing method thereof, a supporting electrode of the fuel cell electrode catalyst, and a fuel cell. The fuel cell electrode catalyst supports the alloy on a carbon support, the alloy comprises a noble metal and a base metal, wherein the molar ratio of the noble metal to the base metal of the above alloy, referring to the ratio of the base metal / the noble metal is set as 4-9.
Owner:SUZUKI MOTOR CORP
Metal material for proton exchange membrane fuel cell cathode catalyst and preparation method thereof
The invention provides a metal material for a proton exchange membrane fuel cell cathode catalyst. An alloy formed by platinum and gallium has a one-dimensional nanowire structure having the length of40-80nm and the diameter of 1-2nm, is provide. The invention also provides a preparation method of the metal material. Firstly Pt nanowires are obtained by reduction of the divalent metal platinum under the organic liquid condition, and a trivalent metal gallium compound and a reducing agent are added into the Pt nanowires and the alloy of Pt and Ga with the one-dimensional nanowire structure isobtained through reduction reaction. The structure of the Pt-Ga alloy material is the one-dimensional nanowire structure, the mass activity of the catalyst supported by carbon is more than nine timesof that of the Platinum-carbon nano-catalyst, the area activity of the catalyst is more than seven times than that of the Platinum-carbon nano-catalyst and the mass activity performance loss of the catalyst is only 15.7% when it is recycled 30000 times in oxygen atmosphere. Besides, the preparation method can realize high content doping of Ga element to Pt nanowires and the content is controllable, the use of the Pt element can be effectively reduced according to the requirements and the battery cost can be reduced; meanwhile, the reaction condition is mild and operation is simple.
Owner:HUNAN UNIV
Platinum-based monatomic electrocatalytic material as well as preparation method and application thereof
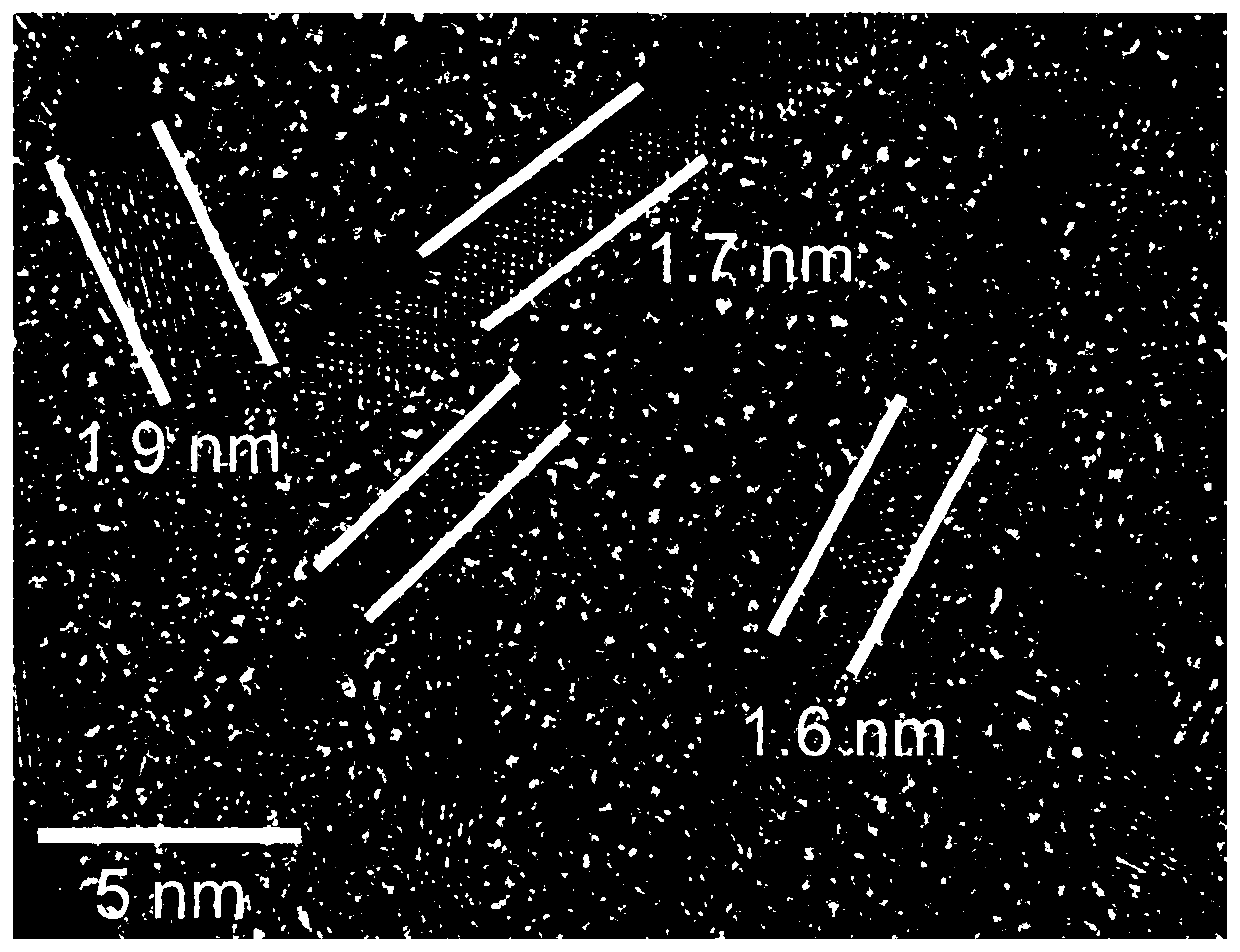
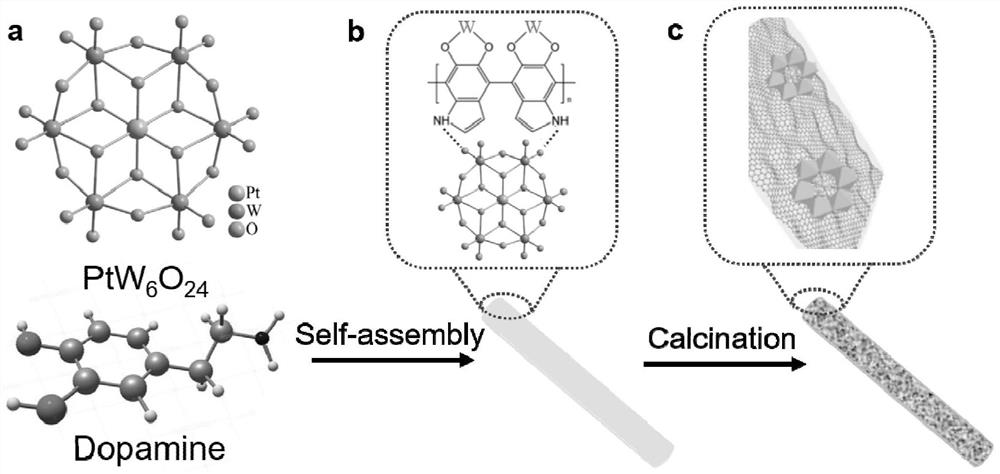
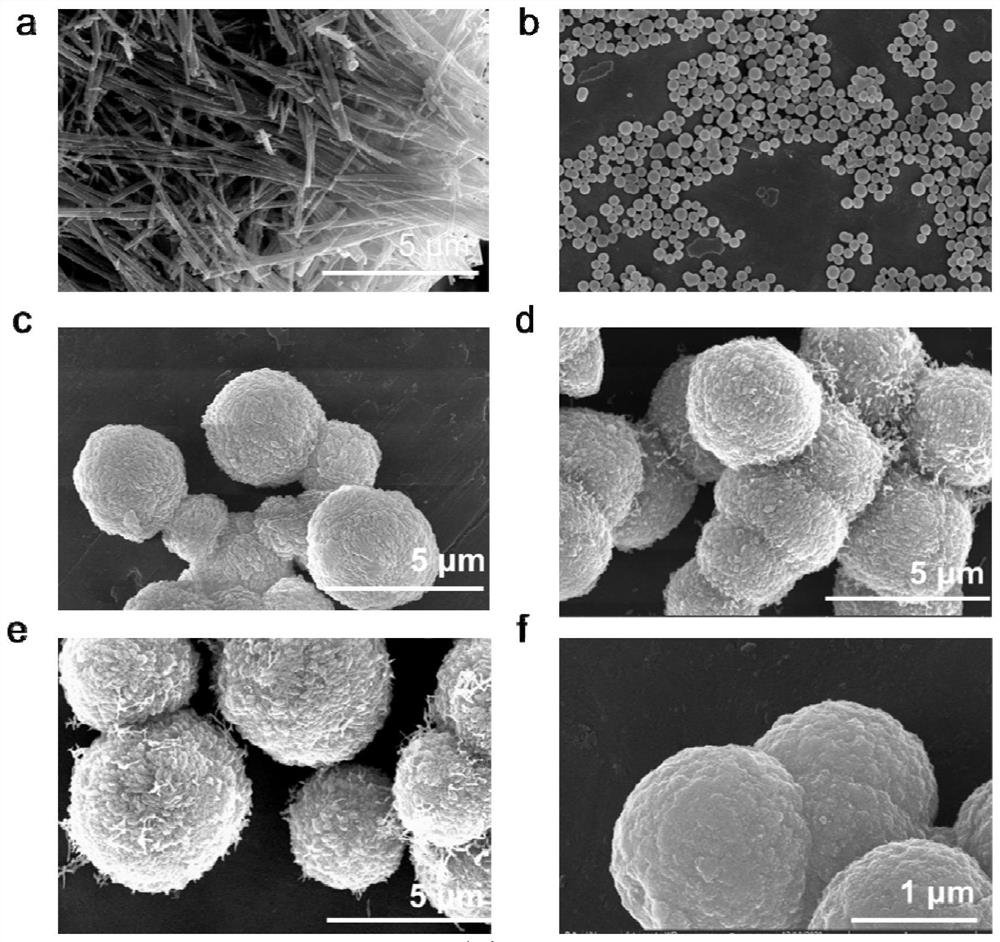
ActiveCN113842936AEfficient dopingIncrease capacityPhysical/chemical process catalystsCell electrodesPlatinumPtru catalyst
The invention provides a platinum-based monatomic electrocatalytic material as well as a preparation method and application thereof, belonging to the field of electrocatalysis. The platinum-based monatomic electrocatalytic material is prepared by taking an Anderson type heteropolyacid compound, dopamine or a salt thereof as raw materials, wherein the Anderson type heteropolyacid compound is Anderson type heteropolyacid or a hydrate thereof, and a salt of the Anderson type heteropolyacid or a hydrate thereof; and the structure of the Anderson type heteropolyacid is H8PtR6O24, and R is a transition metal. The electrocatalytic hydrogen evolution performance of the platinum-based monatomic electrocatalyst in an acid solution is equivalent to the electrocatalytic hydrogen evolution performance of commercial platinum carbon, and the electrocatalytic hydrogen evolution performance of the platinum-based monatomic electrocatalyst in an alkaline solution is remarkably higher than the electrocatalytic hydrogen evolution performance of the commercial platinum carbon. Meanwhile, the long-term durability of the platinum-based monatomic electrocatalyst in the acid solution and the alkaline solution is remarkably superior to the long-term durability of commercial platinum carbon, and the mass activity and the catalytic conversion frequency of the platinum-based monatomic electrocatalyst are also remarkably superior to the mass activity and the catalytic conversion frequency of the commercial platinum carbon. The platinum-based monatomic electrocatalyst prepared in the invention has high activity and long-term stable electrocatalytic performance in a wide pH range, and has wide application prospects.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Metal nitrogen-carbon loaded low-platinum ordered alloy composite catalyst and preparation method thereof
PendingCN114243037AReduce loadEnhanced interactionCell electrodesSolid electrolyte fuel cellsPlatinumPtru catalyst
The invention discloses a metal nitrogen and carbon loaded low-platinum ordered alloy composite catalyst and a preparation method thereof, the composite catalyst comprises metal nitrogen and carbon and platinum alloy loaded on the metal nitrogen and carbon, the metal element in the metal nitrogen and carbon is one or more of iron, cobalt, nickel, copper and manganese; the platinum alloy comprises 2.0-8.6% of platinum and the balance of one or more elements of iron, cobalt, nickel, copper and manganese. The low-platinum-loading-capacity platinum-based ordered alloy loaded on metal nitrogen carbon is successfully prepared by regulating and controlling parameters such as heat treatment temperature, a nitrogen source and a carbon carrier. The metal nitrogen-carbon loaded platinum-iron ordered alloy composite catalyst prepared by the method shows excellent oxygen reduction activity and stability, the mass activity of the metal nitrogen-carbon loaded platinum-iron ordered alloy composite catalyst under the acidic condition of 0.9 V is 3 times that of commercial platinum-carbon, the mass activity of the metal nitrogen-carbon loaded platinum-iron ordered alloy composite catalyst under the alkaline condition is 6.9 times that of commercial platinum-carbon, and the performance of the metal nitrogen-carbon loaded platinum-iron ordered alloy composite catalyst keeps 82.55% of an initial value after 30000 circles of stability tests. The composite catalyst can be used for preparing a low-platinum membrane electrode of a fuel cell.
Owner:NANJING UNIV
Alloy nanocage catalyst, and preparation method and application thereof
The invention provides an alloy nanocage catalyst, and a preparation method and an application thereof. The catalyst comprises an alloy of noble metal and transition metal, wherein the noble metal includes any one or a combination of at least two of palladium, ruthenium, platinum, gold, rhodium, osmium and iridium; the transition metal includes copper; and the catalyst is of a hollow nanocage structure. According to the catalyst, the transition metal part is used for replacing the noble metal to form the alloy, so that the usage amount of the noble metal is reduced, the noble metal and the transition metal have a synergistic effect, an electronic structure is optimized, and the catalyst stability is enhanced; the catalyst has an octahedral hollow cage-like structure and is stable in structure and large in specific surface area, the usage amount of the noble metal is small, the catalyst cost is greatly reduced, and the application prospect is good; the catalyst is applied to the fuel cell positive catalysis; and the noble metal has the mass activity which can reach about 1,000mA mg<-1>, and the specific activity which can reach about 2.0mA cm<-2>.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Petal-shaped Cu doped PtRu alloy catalyst and preparation method thereof
InactiveCN107123818AIncrease surface stressImprove stabilityCell electrodesMetal/metal-oxides/metal-hydroxide catalystsMethanol fuelAlloy catalyst
The invention relates to a petal-shaped Cu doped PtRu alloy catalyst and a preparation method of the catalyst. The petal-shaped Cu doped PtRu alloy catalyst is in a petal-shaped morphology, Pt, Ru and Cu exist in an alloy form, and Pt and Ru atoms are distributed at an active site. The mass activity of the prepared petal-shaped PtRu / Cu catalyst in a methanol oxidation reaction is at least 3.1 times of commercial Pt / C. The preparation method is simple and easy to operate. The catalyst can be widely applied in many fields of methanol fuel cell catalysts and the like due to excellent methanol oxidation performance.
Owner:WUHAN UNIV OF TECH
Three-dimensional carbon structure loaded GaN catalyst and preparation method thereof
The invention discloses a three-dimensional carbon structure loaded GaN catalyst and a preparation method thereof, and belongs to the technical field of fuel cell catalysts. The method comprises the following steps: 1) carrying out a pretreatment process on carbon-based materials with various structures to obtain three-dimensional carbon structure powder; 2) performing plasma treatment on the three-dimensional structure carbon powder to obtain surface-activated three-dimensional structure carbon powder; 3) preparing a GaN nano material by adopting a microwave plasma chemical vapor deposition system; 4) transferring the GaN nano material to the surface of the foamed nickel electrode, and performing electrostatic adsorption on the three-dimensional carbon structure in an aqueous solution; and 5) annealing the three-dimensional carbon structure adsorbing the GaN nano material to obtain the three-dimensional carbon structure loaded GaN catalyst. The mass activity of the prepared three-dimensional carbon structure loaded GaN catalyst is larger than 100 mA / mgGaN at 0.9 V, and after 10000 circles of aging tests, the mass activity attenuation of the three-dimensional carbon structure loaded GaN catalyst is smaller than 20%.
Owner:BEIJING UNIV OF TECH
Platinum-cobalt alloy catalyst for fuel cell, and preparation method thereof
PendingCN113161563ARaise the half-wave potentialHigh densityMaterial nanotechnologyCell electrodesPtru catalystMaterials science
The invention provides a platinum-cobalt alloy catalyst for a fuel cell, and a preparation method thereof. The platinum-cobalt alloy catalyst is prepared by adopting a polyol reduction method, and the preparation method specifically comprises the following steps: firstly, respectively adding a platinum precursor, a cobalt precursor, a carbon carrier and a reducing agent into polyol, carrying out ultrasonic dispersion to obtain a uniform solution, then transferring carbon carrier slurry into a round-bottom flask, respectively dropwise adding the platinum precursor, cobalt precursor and reducing agent solution, carrying out oil bath heating reaction, cooling, centrifuging, washing and carrying out vacuum drying; and grinding, adding an acid solution for etching, cooling, centrifuging, washing, and carrying out vacuum drying to obtain the high-performance platinum-cobalt alloy catalyst. The preparation method is simple in process, low in raw material cost and high in synthesis efficiency, the prepared carbon-loaded platinum-cobalt alloy electrocatalyst is excellent in oxygen reduction reaction activity, and a test result shows that the half-wave potential, the limiting current density, the electrochemical active area, the mass activity and the like of the synthesized platinum-cobalt alloy electrocatalyst are remarkably superior to those of commercial platinum-carbon.
Owner:郑州中科新兴产业技术研究院 +1
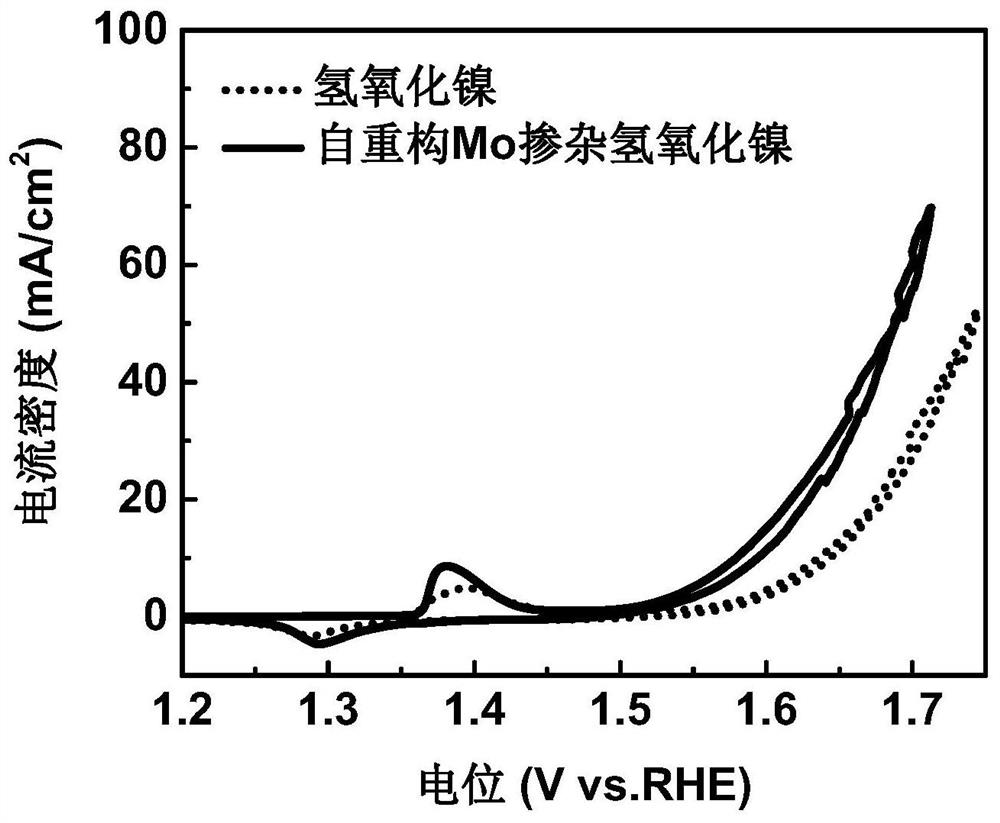
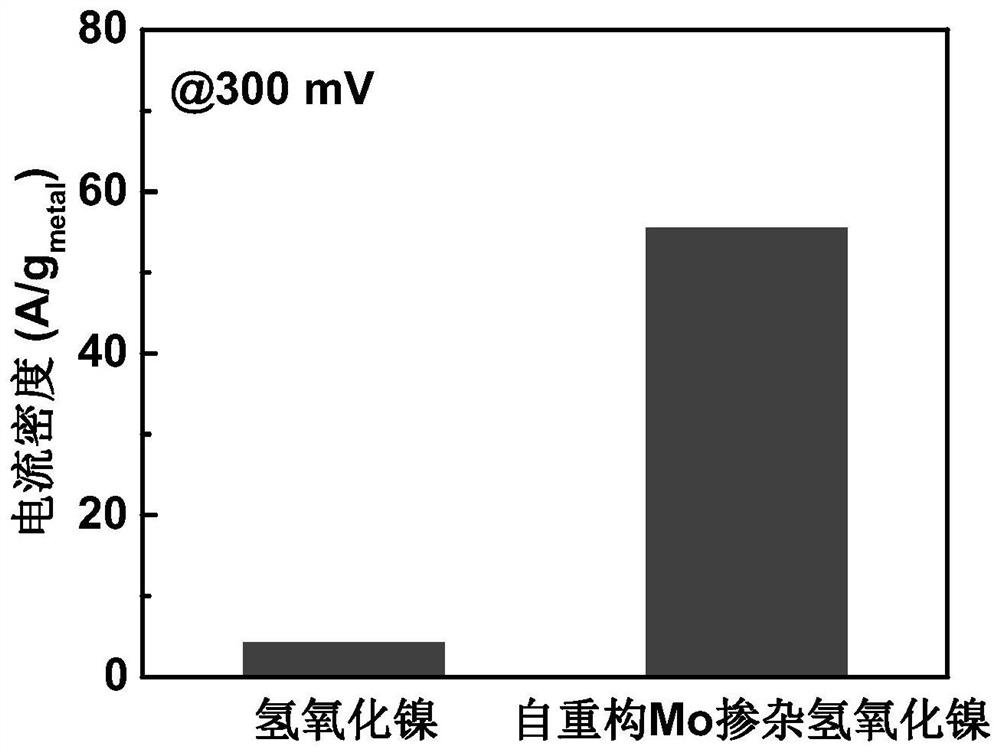
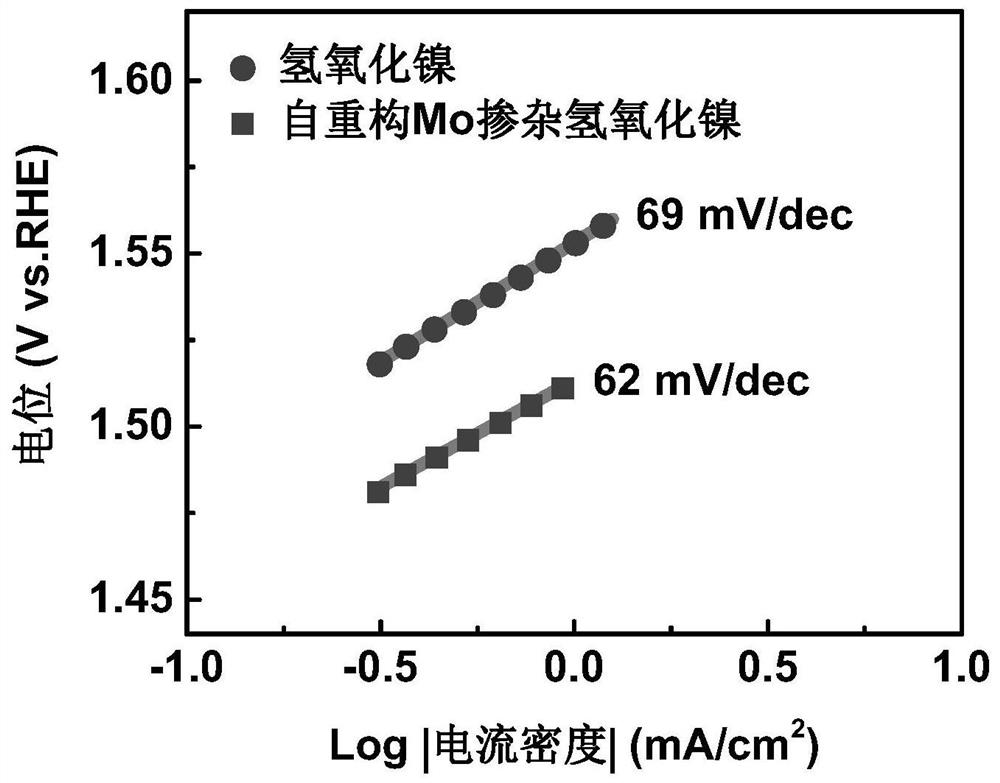
Mo-doped transition metal hydroxide electrocatalyst constructed through deep self-reconstruction as well as preparation method and application of Mo-doped transition metal hydroxide electrocatalyst
ActiveCN113235122AReduce manufacturing costRaw materials are cheapElectrodesPtru catalystOxygen evolution
The invention discloses a Mo-doped transition metal hydroxide electrocatalyst constructed through deep self-reconstruction and a preparation method and application of the Mo-doped transition metal hydroxide electrocatalyst. The method comprises the following steps: activating a MoS2 nanosheet array under an overpotential condition, soaking the MoS2 nanosheet array in a transition metal salt solution for ion adsorption, and performing deep self-reconstruction by a cyclic voltammetry scanning method to obtain the catalyst. According to the method, raw materials are low in price, high-temperature sintering is not needed, energy consumption in the production process is low, and the production cost is low; and according to the method, a transition metal ion adsorption strategy and an electrochemical self-reconstruction strategy are adopted, the preparation process is simple, and the method is suitable for large-scale production. The deep self-reconstruction Mo-doped transition metal hydroxide electrocatalyst provided by the invention has excellent oxygen evolution reaction intrinsic activity, the overpotential under the current density of 10mA / cm<2> is 242mV, and the mass activity current density under the overpotential of 300mV is 1910A / g.
Owner:SOUTH CHINA UNIV OF TECH
Preparation of bifunctional catalyst diatomite domain limited cobalt-platinum-based composite material, and application of bifunctional catalyst diatomite domain limited cobalt-platinum-based composite material in electrocatalytic oxygen reduction reaction and oxygen evolution reaction
InactiveCN107342425AAvoid consumptionEasy to prepareFuel and primary cellsCell electrodesPlatinumOxygen
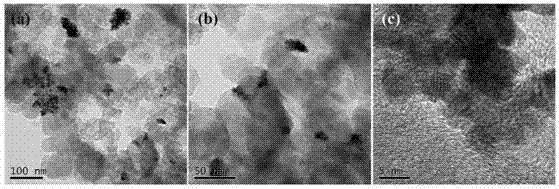
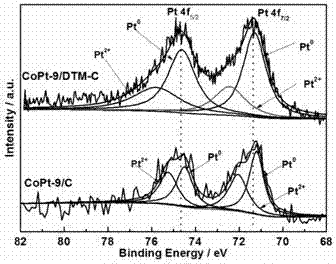
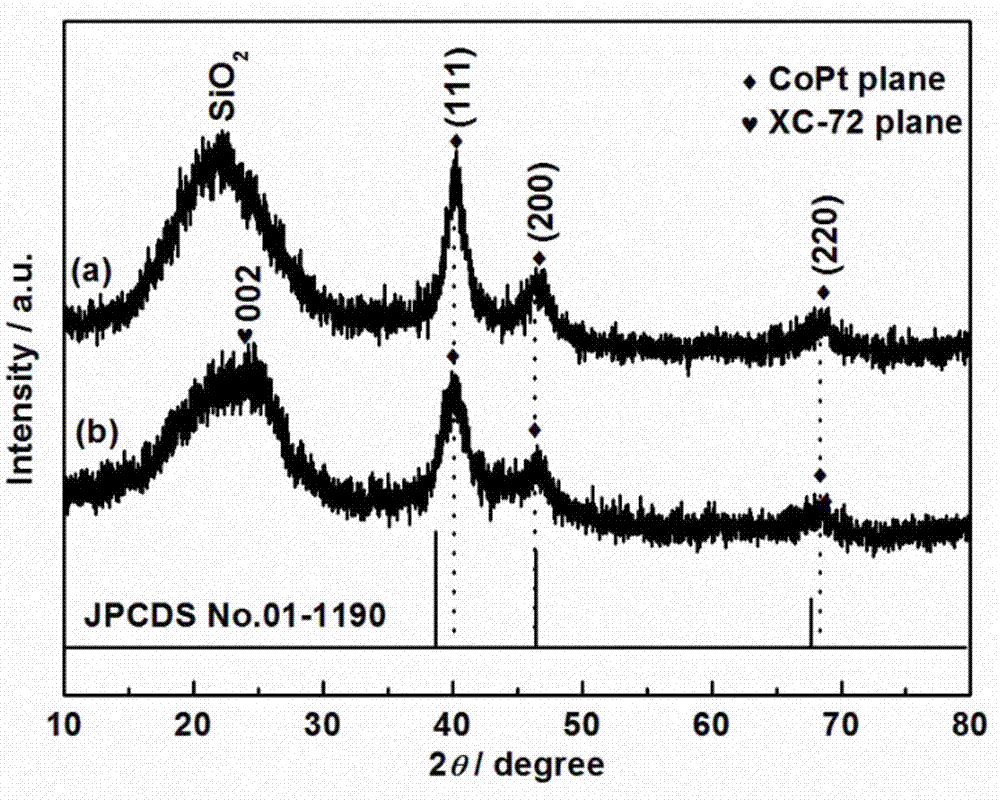
The invention discloses preparation of a diatomite (DTM) domain limited cobalt-platinum-based composite material (CoPt-x / DTM-C) prepared by a simple method, and electrocatalytic application thereof. The invention has the advantages that: (1) the preparation method is simple: firstly, Pt4 and Co2+ ions are adsorbed by the pores of DTM, then the Pt4 and Co2+ ions are reduced, and the structure of the catalyst prepared by the method cannot be damaged easily in the reaction; (2) the catalytic activity is high: in the oxygen reduction reaction, the mass activity and specific activity of the composite material are respectively 2.5 and 1.5 times as large as those of CoPt-x / C and are respectively 4.6 and 2.2 times as large as those of platinum-carbon; and in the oxygen evolution reaction, the overpotential of CoPt-x / DTM-C is reduced by 30 mV compared with that of the CoPt-x / C; and (3) the cost is low: the Pt content of the catalyst is low, the diatomite source is wide, and thus the cost of the catalyst is greatly reduced, and the commercial application prospect is good.
Owner:SOUTH CHINA UNIV OF TECH
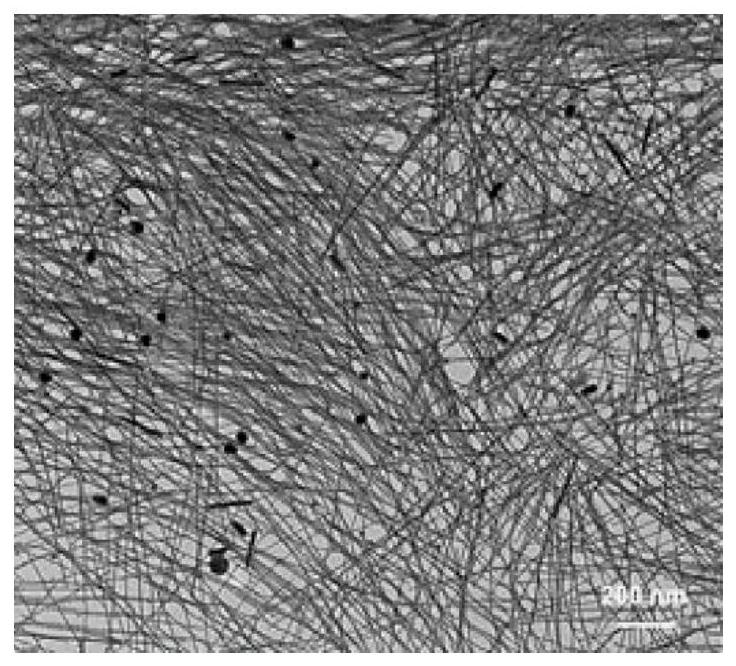
Preparation method of dual-element Pt/PdPt/Pt sandwich tube wall porous nanotube and porous nanotube
The invention provides a porous nanotube with a rough surface and a dual-element Pt / PdPt / Pt interlayer tube wall and a preparation method of the porous nanotube. The Kirkendall effect is used for enabling platinum ions to generate a nucleation phenomenon on the surface of a palladium nanowire, internal palladium is gradually removed through gaps of a deposition layer, and then the hollow PdPt nanotube with the outer diameter being 8.6 nm and the wall thickness being about 2.2 nm is formed; the nanotube is further processed to form an interlayer structure with a tube wall element of Pt / PdPt / Pt. According to the product PdPt nanotube, the platinum element can be fully exposed on the inner side and the outer side of the nanotube wall, the utilization rate of platinum in the material is greatly increased, the tube wall interlayer contains the PdPt double elements, and the catalytic reaction activity is greatly improved through the stress and strain effect between the double metals. When the catalyst is used as a fuel cell cathode reaction catalyst, the reaction activity of the catalyst is greatly improved in an oxygen reduction reaction, the active specific surface area ECSA of the catalyst is 1.46 times that of commercial Pt / C sold in the market, the mass activity (MA) of the catalyst is 14.3 times that of the commercial Pt / C, and the actual specific activity (SA) of the catalyst is 9.64 times that of the commercial Pt / C.
Owner:YANSHAN UNIV
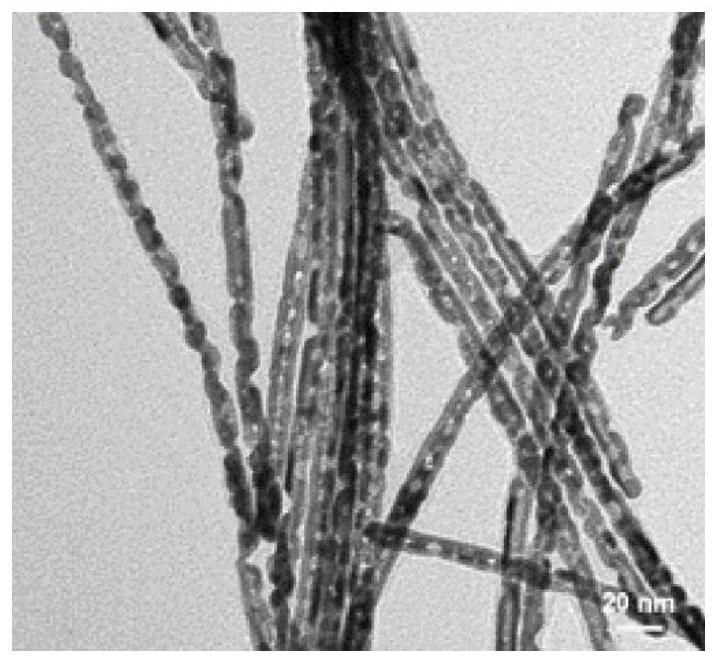
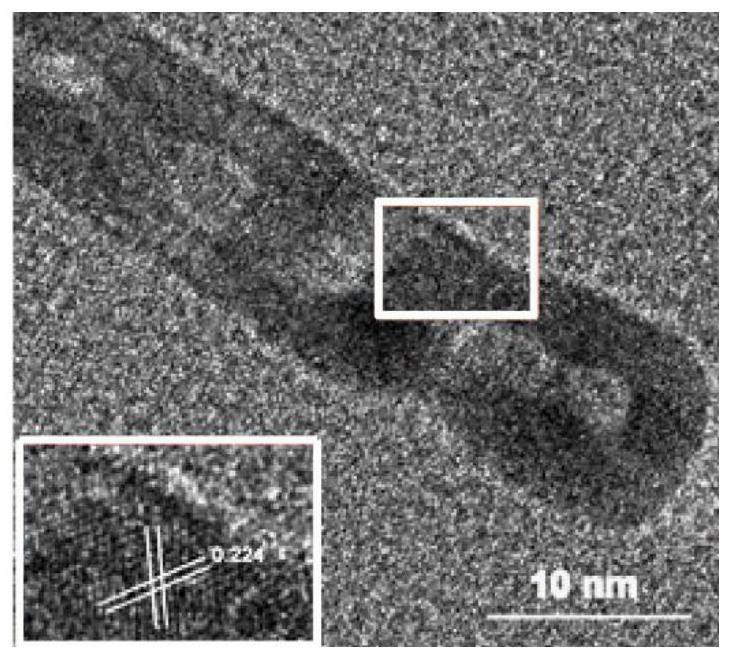
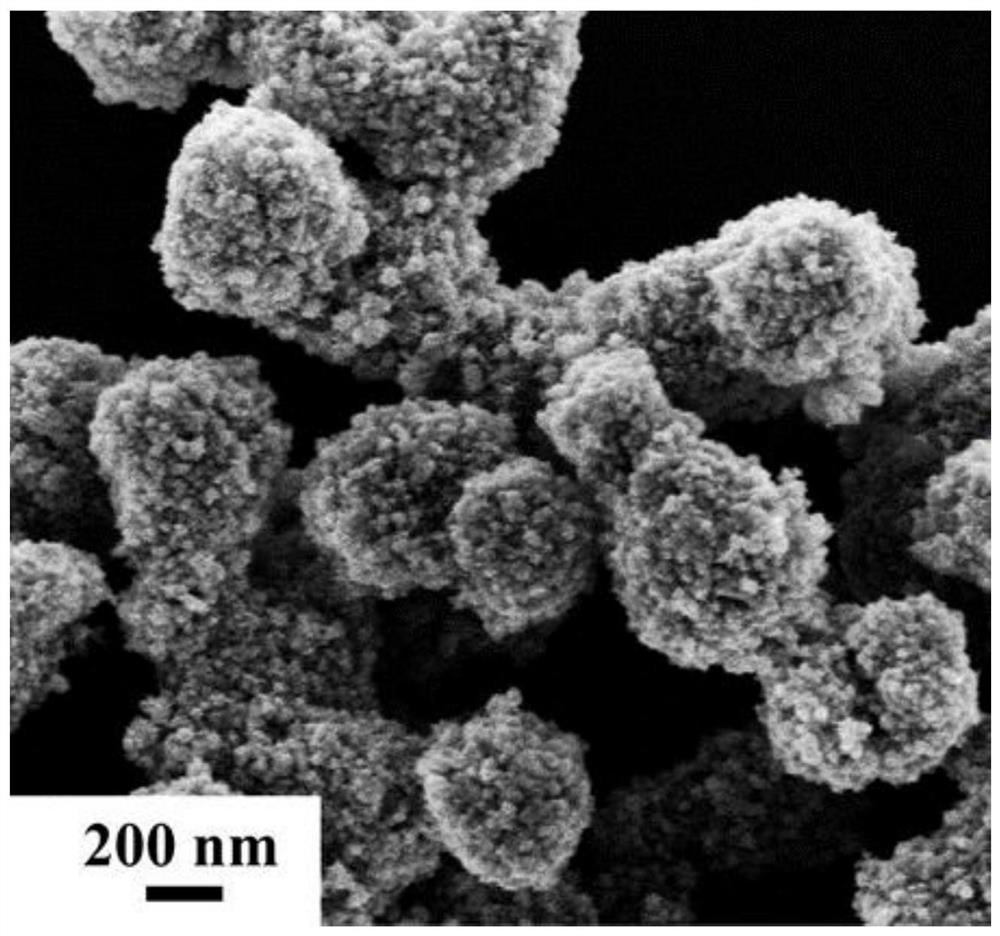
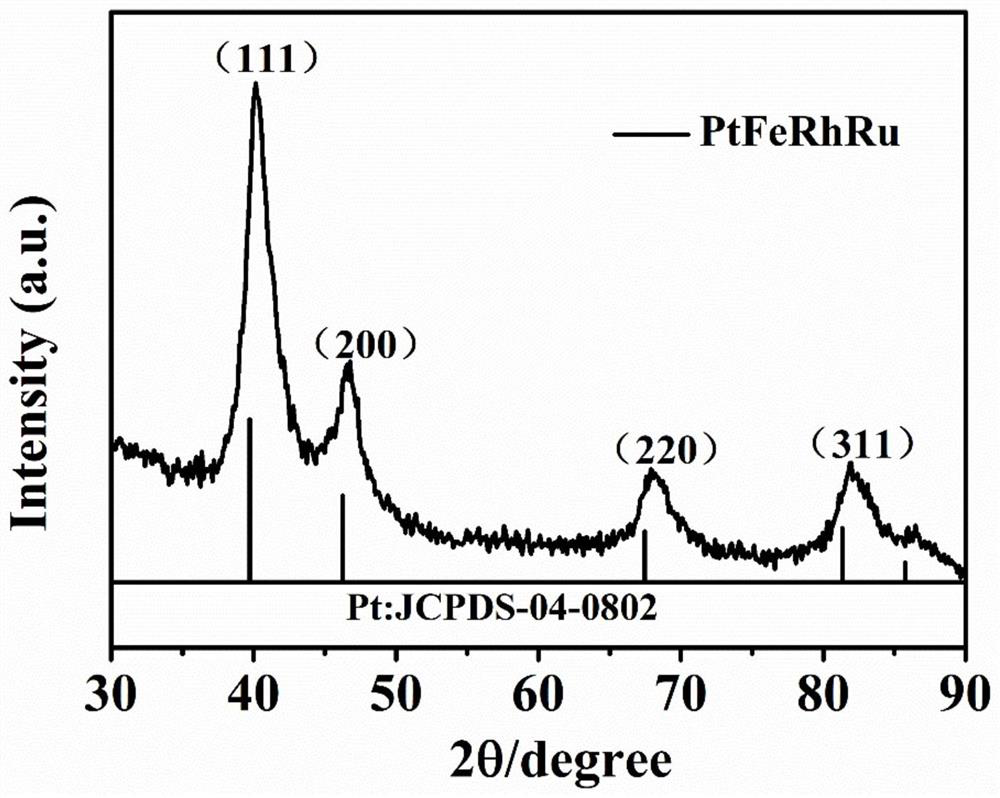
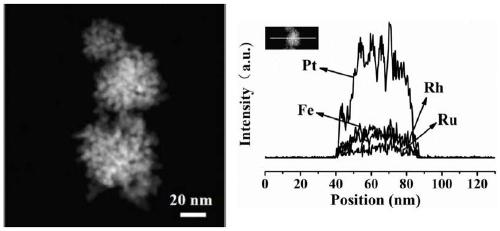
Synthesis and application of quaternary platinum-iron-rhodium-ruthenium nano-alloy with hierarchical structure
ActiveCN111617774ANovel structureImprove catalytic performanceMaterial nanotechnologyTransportation and packagingPlatinumMetallurgy
The invention discloses synthesis and application of a quaternary platinum-iron-rhodium-ruthenium nano-alloy with a hierarchical structure. The synthesis is characterized in that the quaternary platinum-iron-rhodium-ruthenium nano-alloy with the hierarchical structure is novel in morphology and uniform in size, and the hierarchical structure formed by assembling 42-62 nm quaternary platinum-iron-rhodium-ruthenium nano-alloy monomers. In an alkaline hydrogen evolution reaction, the mass activity at the voltage of -0.07 V is 4.44 A mgpt <-1 >, and the specific activity at the voltage of -0.07 Vis 11.33 mA cm <-2 >, and the mass activity at the voltage of -0.07 V and the specific activity at the voltage of -0.07 V are 6.16 times and 11.9 times that of commercial carbon supported platinum respectively. In a stability test, after the current density of the quaternary platinum-iron-rhodium-ruthenium nano-alloy with the hierarchical structure is kept at 10 mA cm <-2 > for 20 hours, the massactivity is only reduced by 5% compared with the initial mass activity, and the commercial carbon-loaded platinum is reduced by 44%.
Owner:GUIZHOU UNIV
Substrate selection for catalyst
InactiveCN103252257AOptimal selection windowWiden selection windowCatalyst carriersCatalyst activation/preparationPlatinumHigh current density
In one embodiment, a method of forming a catalyst / substrate construction includes: identifying a catalyst having a specific activity, determining a surface area factor for supporting the catalyst based on the specific activity of the catalyst; selecting a substrate having the surface area factor; and applying the substrate to the catalyst to form the catalyst / substrate construction. In certain instances, the surface area factor may be determined according to the following equation: SA support ( cm support 2 / cm planar 2 ) = [ '' Baseline '' ( A / mg Pt ) Mass Activity I F Loading ( mg Pt / cm 2 ) ] [ Specific Activity ( [mu] A / cm 2 ) 0.000001 ( A / [mu]A ) ] wherein the term "Baseline" refers to mass activity of 100 A per gram of platinum (Pt) for a comparative catalyst 5 nm Pt nano-particles dispersed on a carbon black support, the term "Mass Activity IF" refers to the activity required to achieve a high current density performance target of 1.5 A / cm2 at 0.67 V, at a platinum loading of 0.1 mg Pt / cm2. In another embodiment, the method also comprises a target superficial area corresponding to a superficial area factor calculated according to the following equation, wherein [sigma] represents catalyst layer thickness, [rho] represents substrate block density, and [u] represents catalyst use ratio percentage.
Owner:FORD GLOBAL TECH LLC
Inorganic-organic core-shell framework loaded low-dose precious metal palladium material and preparation thereof and application in electro-catalytic dechlorination hydrogenation reaction
ActiveCN113846342AAdjustable sizeIncrease surface areaElectrolytic organic productionElectrodesOrganic compoundMaterials science
The invention discloses an inorganic-organic core-shell framework loaded low-dosage precious metal palladium material and preparation thereof and application of the material in electro-catalytic dechlorination hydrogenation reaction. The preparation method comprises the following steps: polymerizing dopamine around a TiO2 one-dimensional nanorods which vertically grow on the surface of FTO and have an array structure by utilizing an in-situ auto-polymerization effect of the dopamine to form an inorganic-organic core-shell structure of TiO2 nanorod wrapped by the dopamine; and uniformly dispersing and loading an extremely low amount of palladium nanoparticles on the core-shell framework by adopting a chemical impregnation method to obtain an electrode material, wherein the loading capacity of precious metal palladium in the electrode material is only 0.29%, when the electrode material is used as a cathode to be applied to electro-catalysis of a dechlorination reaction of a chlorinated organic compound, the mass activity reaches up to 44.39 min<-1>g<-1>, the conversion rate of dechlorination of the chlorinated organic compound can reach up to 94%, very high electro-catalytic reaction activity is shown, and a relatively large application prospect is achieved in electro-catalytic hydrodechlorination.
Owner:ZHEJIANG UNIV OF TECH
A preparation method of a platinum-based alloy catalyst
ActiveCN109004242AImprove surface structureAvoid churnCell electrodesFinal product manufactureAlloy catalystWorking environment
The invention discloses a preparation method of a platinum-based alloy catalyst, which comprises the following steps: (1) preparing alloy particles of platinum and 3d transition metal with nanometer size; (2) carrying out acid treatment on the alloy particles prepared in the step (1); (3) annealing the alloy particles treated in the step (2); The platinum-based alloy catalyst prepared by the invention can prevent the loss of transition metal in the working environment, The electrochemical active area, oxygen reduction specific activity and oxygen reduction mass activity of the platinum-based alloy catalyst are basically kept unchanged in the long-term use process, which ensures the long-term durable use of the platinum-based alloy catalyst and is suitable for large-scale industrial production.
Owner:QINGDAO CHUANGQI XINNENG CATALYSIS TECH CO LTD
Multi-element doped platinum-based catalyst and preparation method and application thereof
InactiveCN111224118AIncreased durabilityIncrease profitMaterial nanotechnologyCell electrodesPtru catalystTest battery
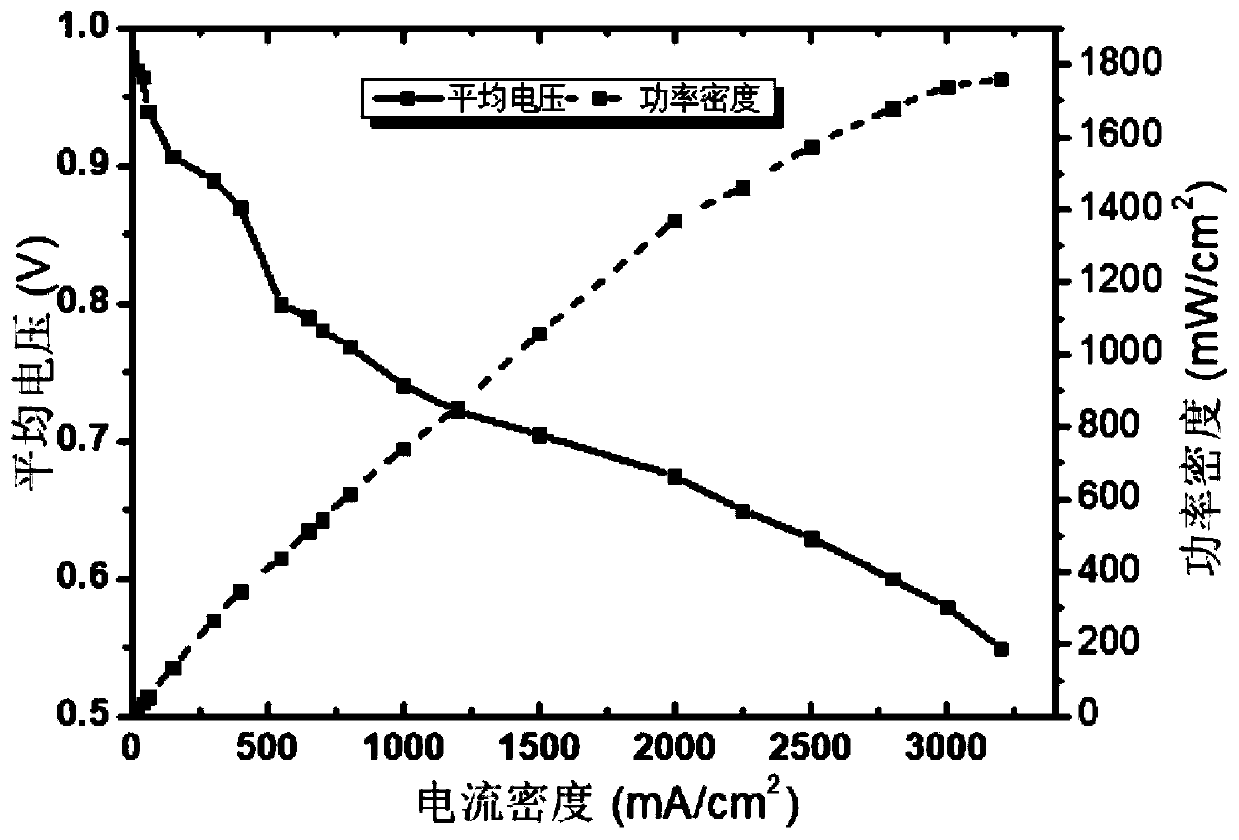
The invention relates to a multi-element doped platinum-based catalyst and a preparation method and application thereof. According to the preparation method of the platinum-based catalyst, combining an ion adsorption process and a top-speed reduction process, and efficiently and rapidly forming the doped platinum-based nanocrystalline formed on the surface of the carbon material. The method has the advantages of being easy to operate and control and suitable for engineering mass production; the multielement doped platinum-based catalyst prepared by the method disclosed by the invention is highin ORR activity, and the mass activity is as high as 255 mA / mg@0.9 V; the catalyst is prepared into a CCM and the CCM is assembled into a single battery for testing, wherein the active area is 50 cm<2>, the test battery temperature is 75 DEG C, the humidification temperature is 70 DEG C, the relative humidity is 60%RH and the stoichiometric ratio hydrogen / air is 1.2 / 2.5, the current density can reach 2,500 mA / cm<2> and 0.63 V, and the peak power density can reach 1.6 W / cm <2>.
Owner:FAW JIEFANG AUTOMOTIVE CO
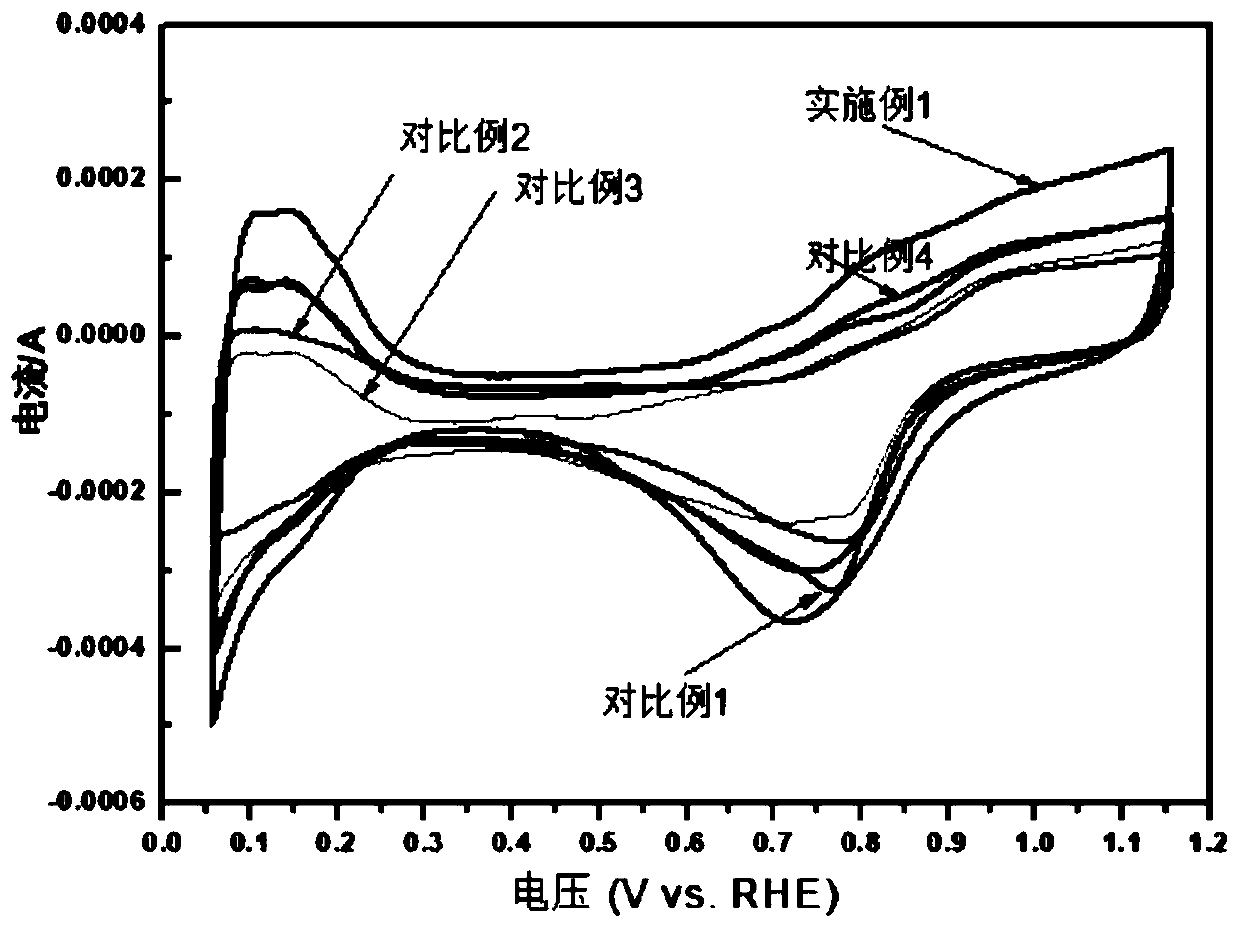
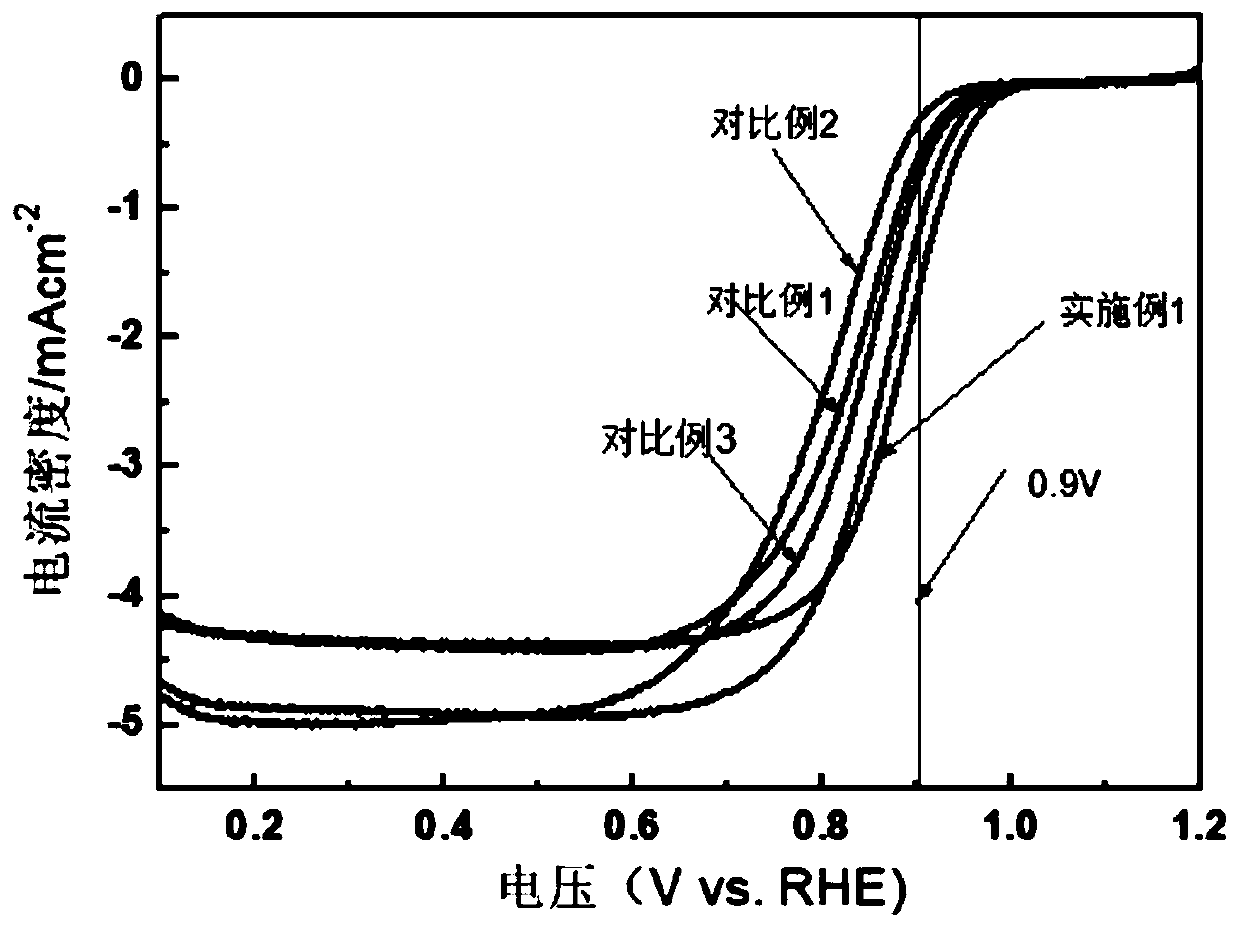
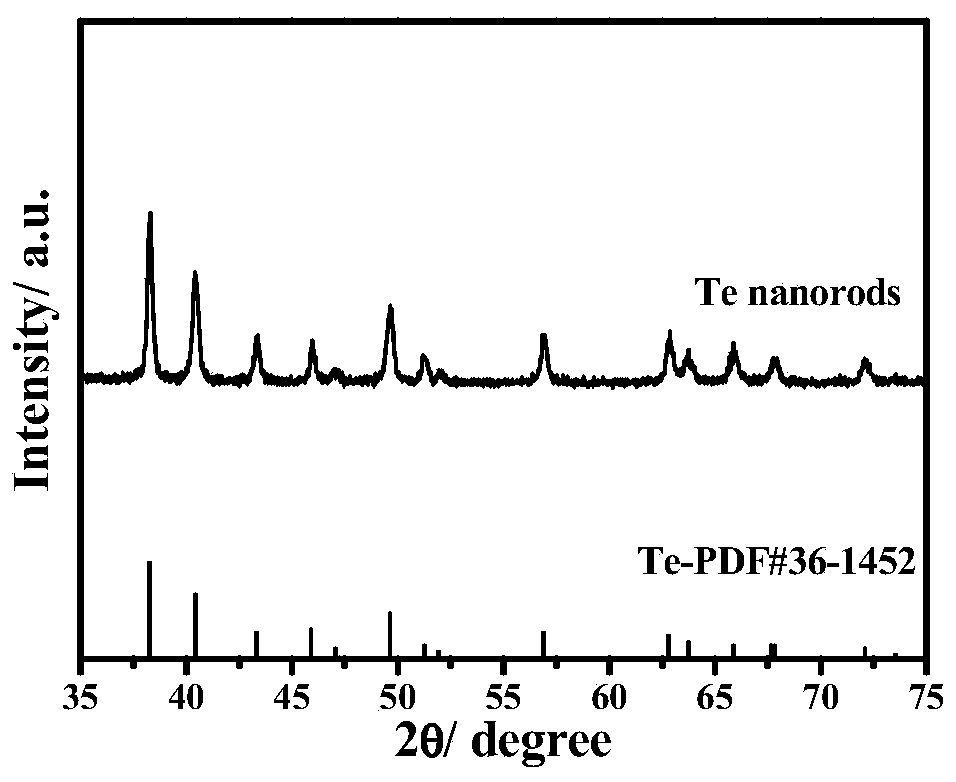
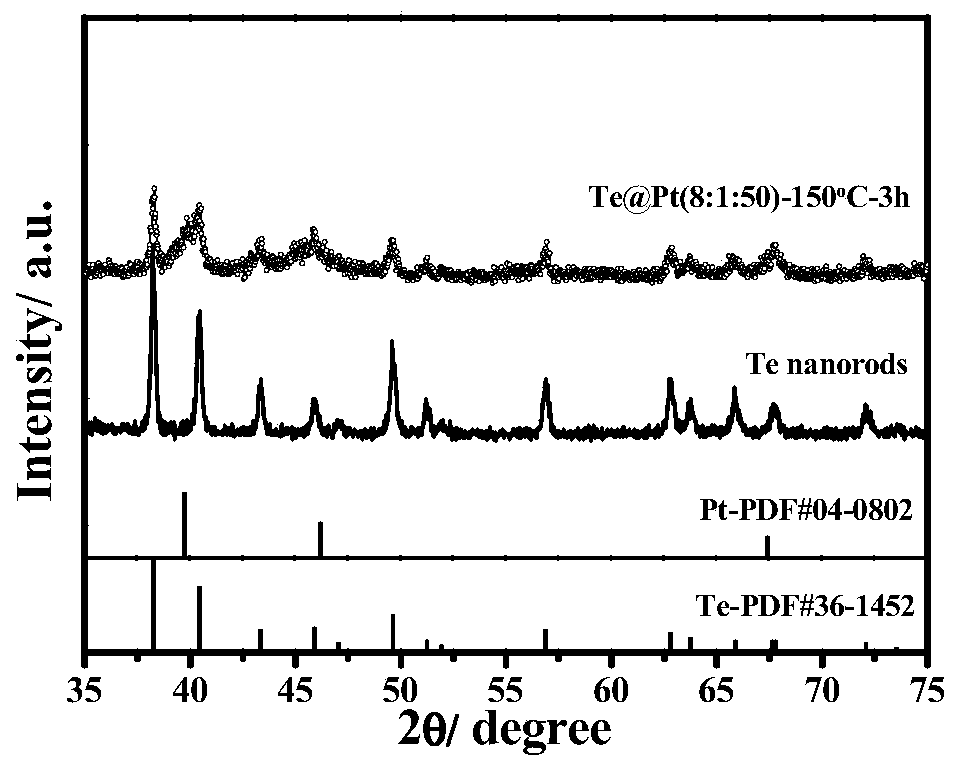
Preparation method of core-shell structure Te @ metal electrooxidation catalyst
InactiveCN110061246AIncrease profitMany active sitesCell electrodesNanotechnologyAlloyPt based catalysts
The invention discloses a preparation method of a core-shell structure Te @ metal electrooxidation catalyst. According to the method, a hard template Te is taken as a core, and noble metal Pt, Pd or an alloy thereof are taken as a shell, and polyvinylpyrrolidone is added, so as to obtain the core-shell structure Te @ metal electrooxidation catalyst by adopting an oil bath heating mode. The Te @ metal electrooxidation catalyst disclosed by the invention is relatively low in noble metal content and has excellent alcohol oxidation performance, wherein the mass activity in the anodic oxidation reaction of a liquid fuel cell is far higher than that of a commercial Pt-based catalyst, and the catalyst can be applied to the field of liquid fuel cells as a high-efficiency electrocatalyst.
Owner:YANGZHOU UNIV
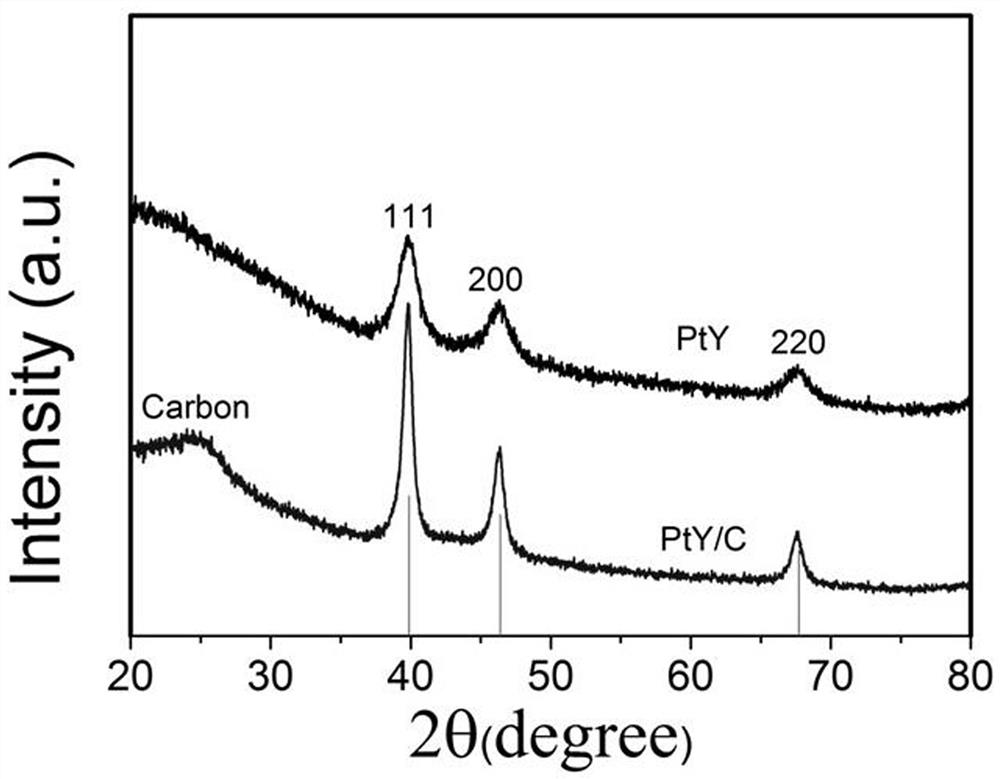
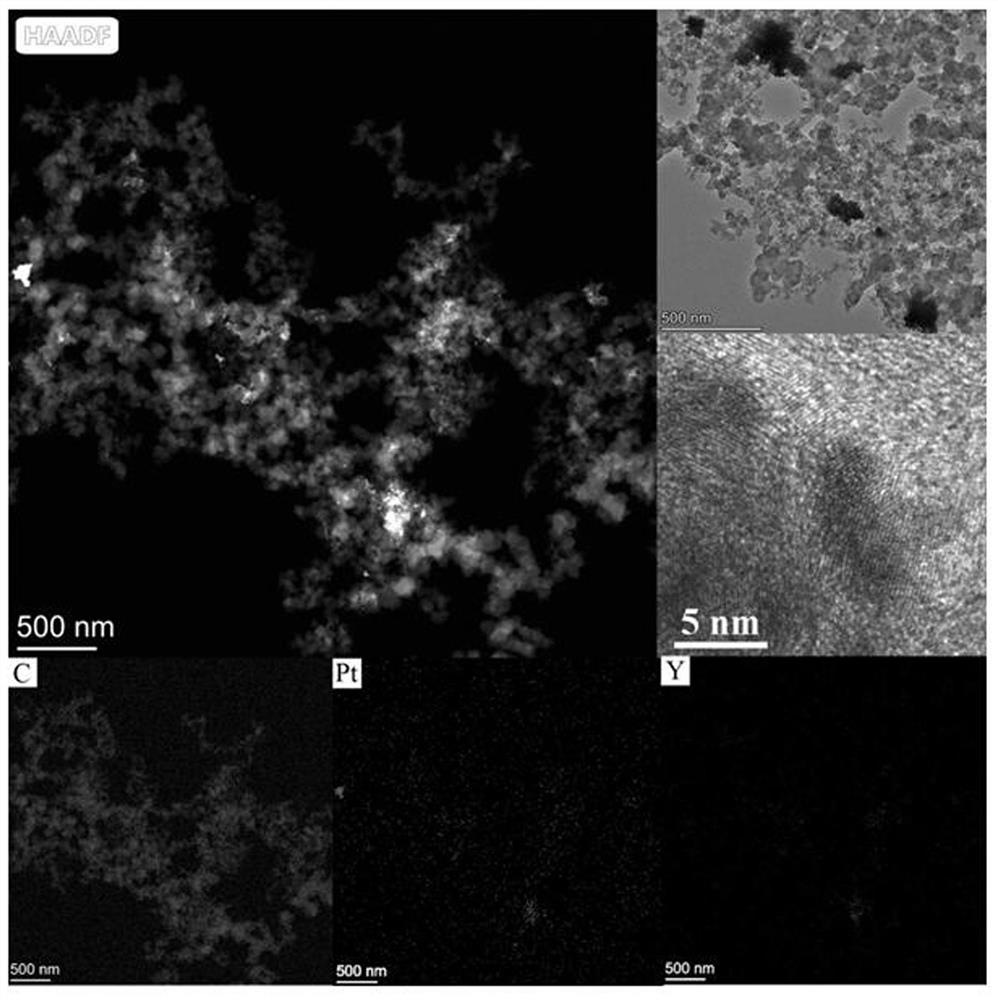
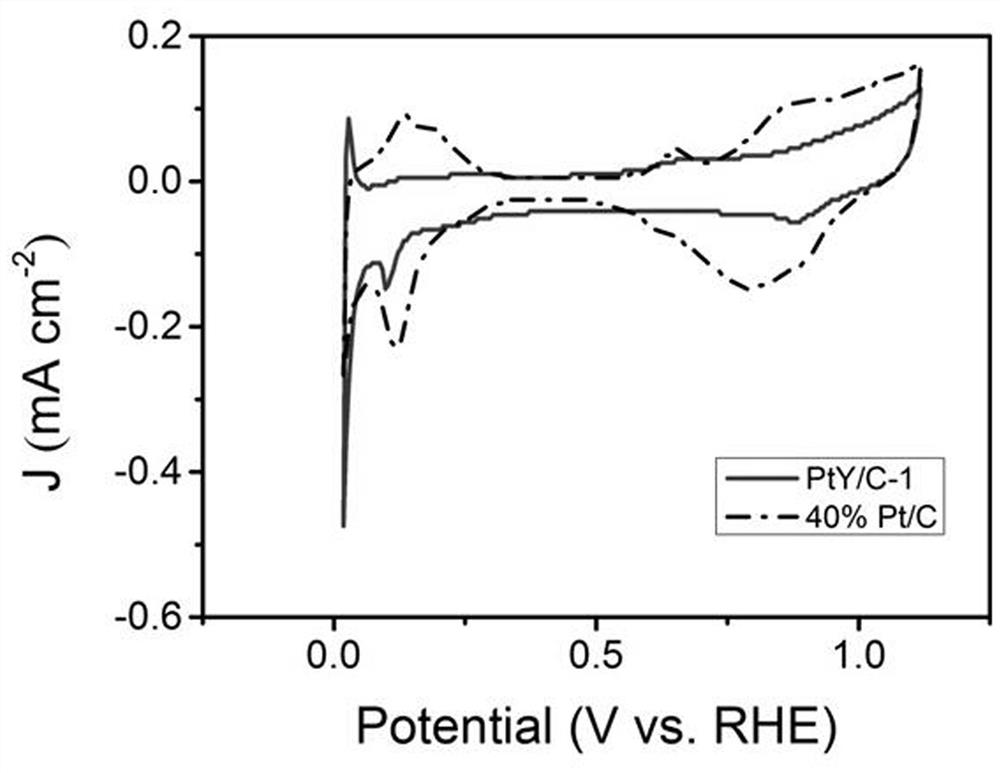
Carbon-loaded platinum-yttrium catalyst and preparation method and application thereof
The present invention discloses a carbon-loaded platinum-yttrium catalyst. Platinum acetylacetonate and yttrium acetylacetonate are used as a platinum source and a yttrium source, a platinum-yttrium material is obtained through a hydrothermal reaction, then the platinum-yttrium catalyst is obtained through carbon loading, and the obtained material has the low platinum content of 6.0-6.5%. The preparation method comprises the following steps: 1) preparing the platinum-yttrium material; and 2) preparing the carbon-loaded platinum-yttrium catalyst. When the carbon-loaded platinum-yttrium catalystis used as a fuel cell catalyst, the half-wave potential is 0.863 V, the mass activity is 0.09 Amg pt-1@0.9 V, and the electrochemical active surface area is 43-45 m2gpt-1. The whole technological process is simple, clean, environmentally friendly and free of danger, solves the problem of high raw material price, reduces platinum loading capacity and has excellent catalytic performance.
Owner:GUILIN UNIV OF ELECTRONIC TECH
Layered platinum on freestanding palladium nano-substrates for electrocatalytic applications and methods of making thereof
ActiveUS11114671B2Polycrystalline material growthFrom normal temperature solutionsPlatinumPtru catalyst
Core-shell nanostructures with platinum overlayers conformally coating palladium nano-substrate cores and facile solution-based methods for the preparation of such core-shell nanostructures are described herein. The obtained Pd@Pt core-shell nanocatalysts showed enhanced specific and mass activities towards oxygen reduction, compared to a commercial Pt / C catalyst.
Owner:GEORGIA TECH RES CORP
High-efficiency doping method of doped NbOx platinum-based catalyst used for fuel cell catalysts
ActiveCN106334553AImprove physical and chemical propertiesImprove conductivityCell electrodesMetal/metal-oxides/metal-hydroxide catalystsFuel cellsFiltration
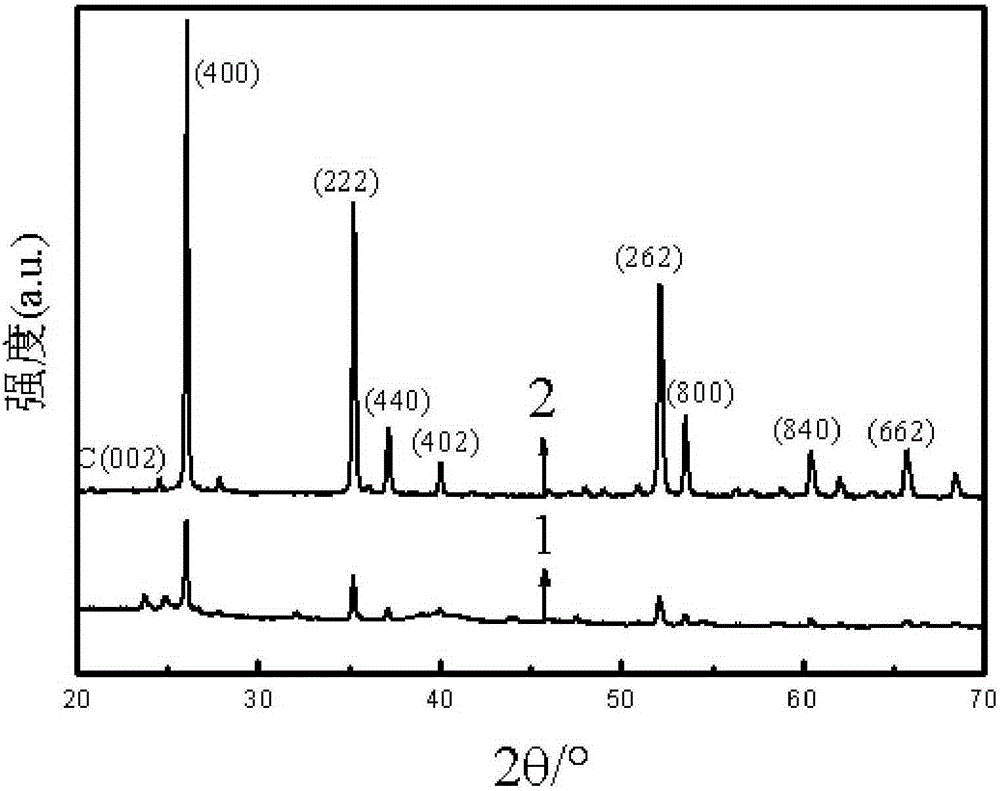
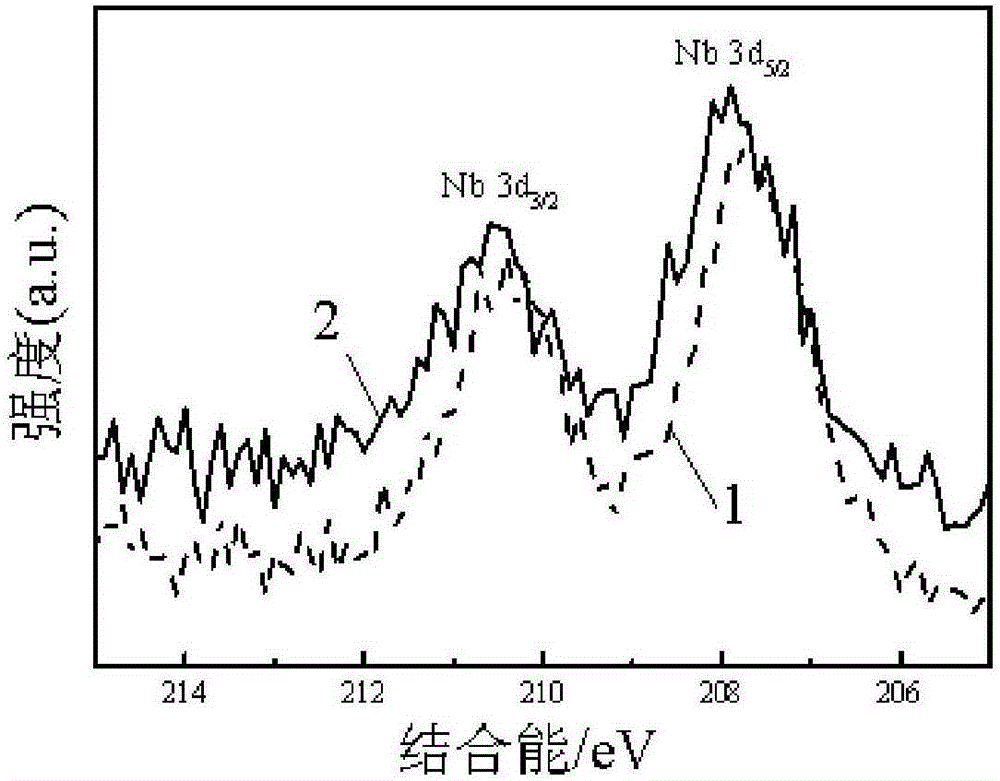
The invention discloses a high-efficiency doping method of a doped NbOx platinum-based catalyst used for fuel cell catalysts, and relates to a preparation method of doped NbOx powder. The purpose of the invention is to solve the problems of low conductivity and poor catalysis activity of present NbOx oxides. The method comprises the following steps: 1, preparing a precursor; 2, roasting; 3, doping; and 4, adjusting the pH value of a reaction solution III, carrying out suction filtration, cleaning, and drying to obtain the doped NbOx platinum-based catalyst used for fuel cell catalysts. The half-wave potential of the doped NbOx platinum-based catalyst used for fuel cell catalysts, prepared in the invention, is 0.79-0.86 V, and is more positive than that of a carbon supported platinum catalyst, and the mass activity of the doped NbOx platinum-based catalyst is high, can reach 173.44 mAmgPt<-1> and is 2.96-5.3 times higher than that of the carbon supported platinum catalyst. The doped NbOx platinum-based catalyst used for fuel cell catalysts can be obtained through the high-efficiency doping method.
Owner:HARBIN INST OF TECH
Preparation method and application of platinum-carbon quantum dot/multi-walled carbon nanotube composite material
PendingCN111905722AHER highHigh activityCatalyst activation/preparationElectrolytic organic productionPtru catalystElectrolysis
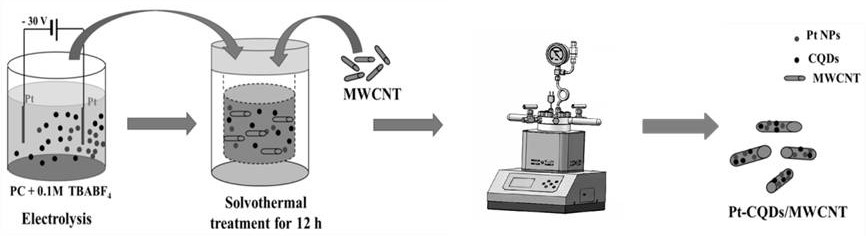
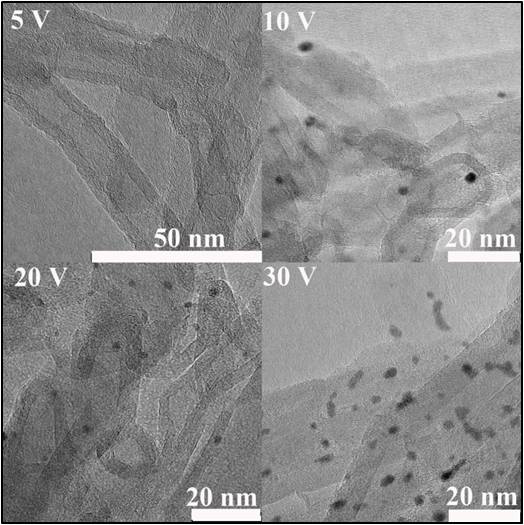
The invention provides a preparation method and application of a platinum-carbon quantum dot / multi-walled carbon nanotube composite material. According to the method, platinum nanoparticles (Pt NP) and carbon quantum dots (CQDs) are prepared in situ under the auxiliary action of an electric field, and the Pt NP and the CQDs are subjected to subsequent thermal adjustment in the process of loading the Pt NP and the tetrabutylammonium hydroxide (TBAOH) by controlling electrolytic voltages (30V, 20V, 10V and 5V) and electrolytes (tetrabutylammonium tetrafluoroborate (TBABF4), tetrafluoride (TBACF3SO3)) and tetrabutylammonium fluoride). Thus, the Pt-CQDs / MWCNT composite catalyst is obtained. The adopted synthesis process is simple, in-situ synthesis is adopted, the prepared platinum-carbon quantum dot / multi-walled nanotube composite material is an electrocatalyst, and the mass activity of a hydrogen evolution reaction (HER) and an ethanol oxidation reaction (EOR) of a PtCQDs / MWCNT catalystis 14.8 times higher than that of commercially available Pt / C (the overpotential is -40 mV) and 5.6 times higher than that of a front peak.
Owner:山西师范大学
A kind of preparation method of platinum-based alloy catalyst
ActiveCN109004242BImprove surface structureAvoid churnFinal product manufactureCell electrodesAlloy catalystWorking environment
Owner:QINGDAO CHUANGQI XINNENG CATALYSIS TECH CO LTD
Ultrahigh-stability oxygen reduction catalyst for room-temperature hydrogen fuel cell
PendingCN114883588AThe synthesis method is simple and mildImprove catalytic performanceCell electrodesTransportation and packagingPlatinumPtru catalyst
The invention discloses an ultrahigh-stability oxygen reduction catalyst for a room-temperature hydrogen fuel cell, which is characterized in that copper-rich octahedral PtCu is adopted as a seed, a PtCu cluster is epitaxially grown on an octahedral PtCu / Pt core-shell structure, and the size of the PtCu cluster is 0.8-2.1 nm. The synthesis method is simple and mild, and an X-ray diffraction spectrum shows that the synthesized PtCu nanoparticles are face-centered cubic alloy. The mass activity of the PtCu alloy in oxygen reduction is 1.42 A mg <-1 >, which is 8.9 times that of commercial carbon-loaded platinum. After 140,000 cycles of accelerated stability test, the activity of the PtCu alloy still keeps 102.1% of the initial activity, while the commercial carbon-loaded platinum is reduced by 43.8% after 10,000 cycles of accelerated stability test.
Owner:GUIZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com