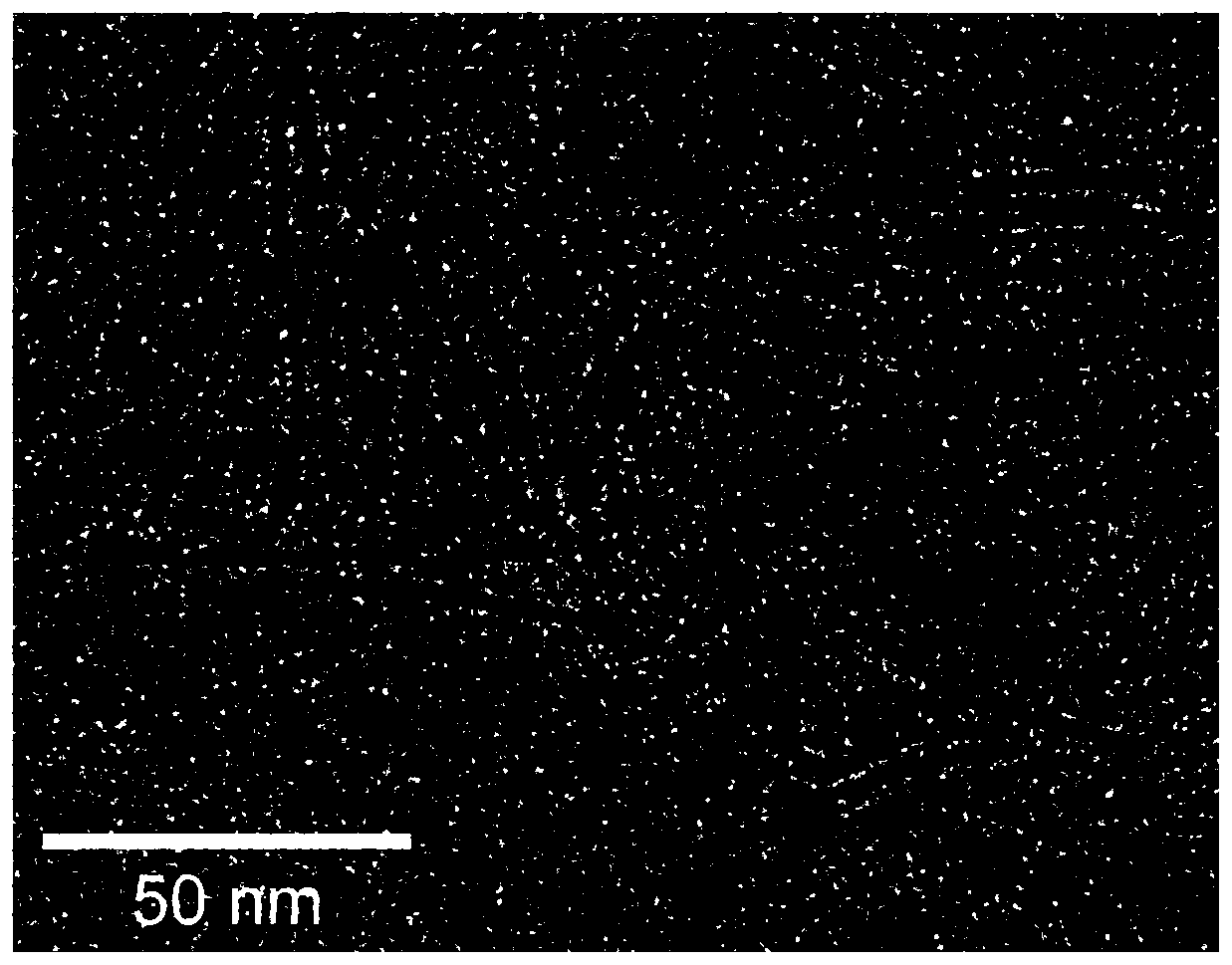
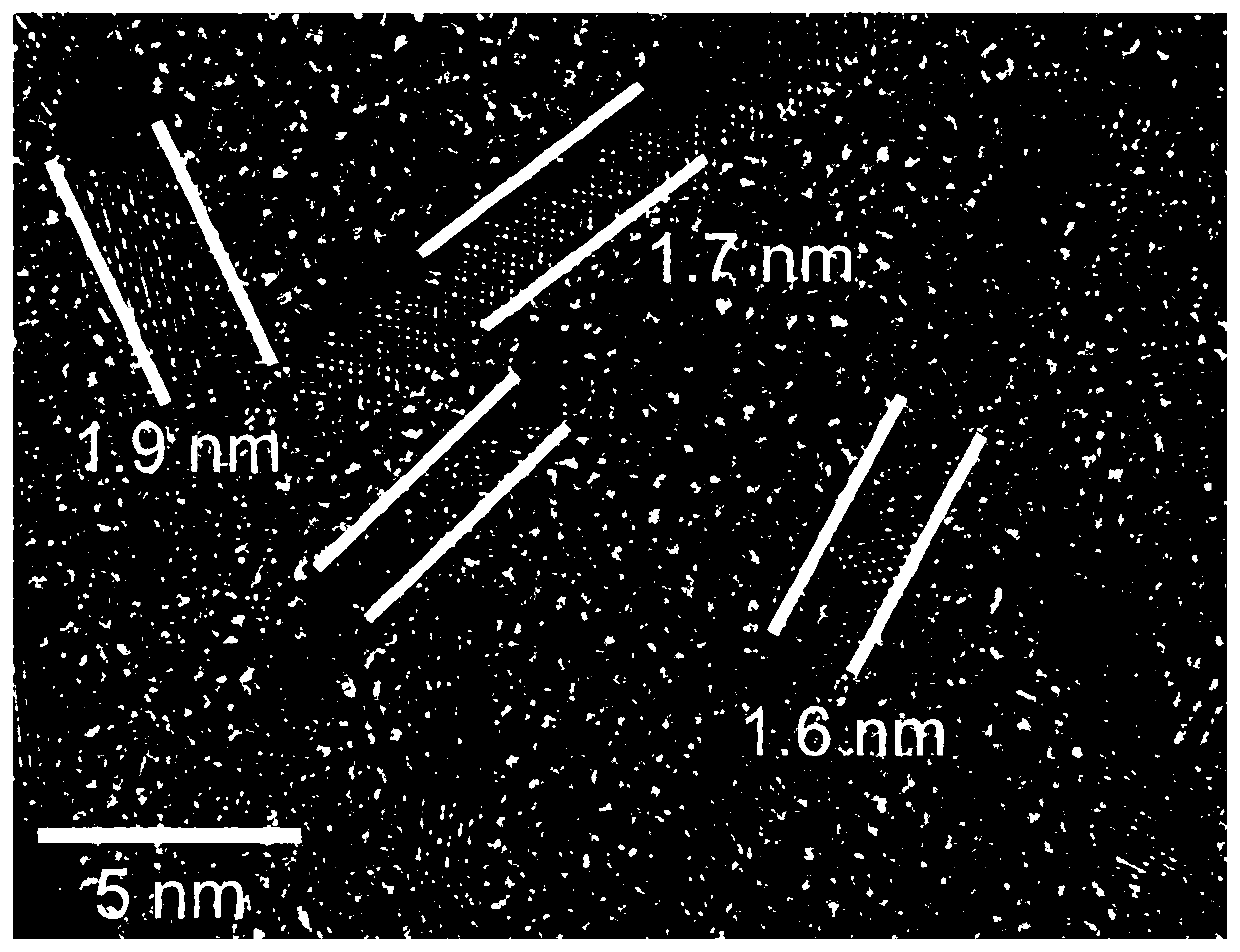
Metal material for proton exchange membrane fuel cell cathode catalyst and preparation method thereof
A fuel cell cathode and proton exchange membrane technology, which is applied in the direction of battery electrodes, circuits, electrical components, etc., can solve the problems of slow reaction kinetics, high battery cost, and poor stability of fuel cells, so as to increase the surface area and reduce battery cost , the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A method for preparing a metal material for a proton exchange membrane fuel cell cathode catalyst, using the following steps:
[0022] Step Ⅰ: Weigh 10 mg of anhydrous platinum acetylacetonate and 90 mg of cetyltrimethylammonium bromide into the first reaction bottle, pipette 4 mL of solvent oleylamine into the bottle, and then sonicate for 30 minutes to make it After forming a homogeneous system, add 15 mg of anhydrous tungsten hexacarbonyl to it, and tighten the cap of the bottle to keep the system in a closed environment. Afterwards, the reaction bottle was placed in an oil bath at 160° C. for 2 h to obtain Pt nanowires.
[0023] Step II: Weigh 36.7 mg of anhydrous gallium acetylacetonate and 100 mg of ascorbic acid into the second reaction bottle, and transfer the system containing Pt nanowires prepared in step I to the second reaction bottle, that is, the molar ratio of platinum and gallium The ratio is 1:4; ultrasonically disperse until the system is uniform, the...
Embodiment 2
[0026] After the Pt-Ga nanowire alloy loaded carbon black prepared in Example 1 is prepared as a catalyst, it is dropped on a glassy carbon electrode, and a rotating disk electrode is used to carry out an oxygen reduction test. The test results of the linear sweep voltammetry curve show that Pt -The half-wave potential of the Ga / C catalyst is 0.95V, much higher than the 0.88V of the Pt / C catalyst as a comparison; the mass activity at the potential of 0.9V is 1.70Amg -1 , which is 9.4 times that of the comparative Pt / C catalyst; the site activity at a potential of 0.9V is 2.0mAcm -2 , which is 7.9 times that of the comparative Pt / C catalyst. After 30,000 cycles, the catalyst of the Pt-Ga nanowire alloy prepared in Example 1 had only 15.6% mass activity loss, while under the same conditions, the mass activity loss of the comparative Pt / C nanocatalyst was 78.2%.
Embodiment 3
[0028] A method for preparing a metal material for a proton exchange membrane fuel cell cathode catalyst, using the following steps:
[0029] Step Ⅰ: Weigh 15mg of anhydrous platinum chloride and 75mg of dodecyldimethylammonium bromide into the first reaction bottle, pipette 5mL of solvent oleylamine into the bottle, and then ultrasonicate for 20min It forms a homogeneous system, and 8 mg of molybdenum hexacarbonyl is added thereto, and the bottle cap is tightened to keep the system in a closed environment. Afterwards, the reaction bottle was placed in an oil bath at 180° C. for 2 h to obtain Pt nanowires.
[0030] Step II: Weigh 27.5 mg of anhydrous gallium chloride and 80 mg of glucose into the second reaction bottle, and transfer the system containing Pt nanowires prepared in step I to the second reaction bottle, that is, the molar ratio of platinum and gallium The ratio is 1:3; ultrasonically disperse until the system is uniform, then put the second reaction bottle into a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com