Double-targeted compound, preparation method and application thereof
A compound and reaction technology, applied in the field of dual-targeting compounds, can solve the problems of poor tumor specificity, insufficient sensitivity, and short imaging time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] Embodiment 1: the preparation of formula 5 compound and formula 7 compound
[0160] The compound of formula 5 can be prepared by following steps (1) and (2):
[0161] (1) Under the protection of inert gas at 0°C, the equivalent of compound 1 and compound 2 were dissolved in methylene chloride and stirred at a low speed, and 1 equivalent of 1-(3-dimethylaminopropyl)-3- Ethylcarbodiimide hydrochloride and a catalytic amount of 4-dimethylaminopyridine in dichloromethane. Stir at a low speed for 24 hours. After the end of the reaction is monitored by silica gel thin-layer chromatography, spin the reaction solvent to dryness, add ethyl acetate, wash with 1mol / L aqueous hydrochloric acid solution, saturated aqueous sodium bicarbonate solution and saturated saline, and anhydrous sodium sulfate. Let dry overnight. Concentrated by filtration, the crude product was directly used in the next reaction;
[0162]
[0163] (2) Under the protection of inert gas at 0°C, the equiva...
Embodiment 2
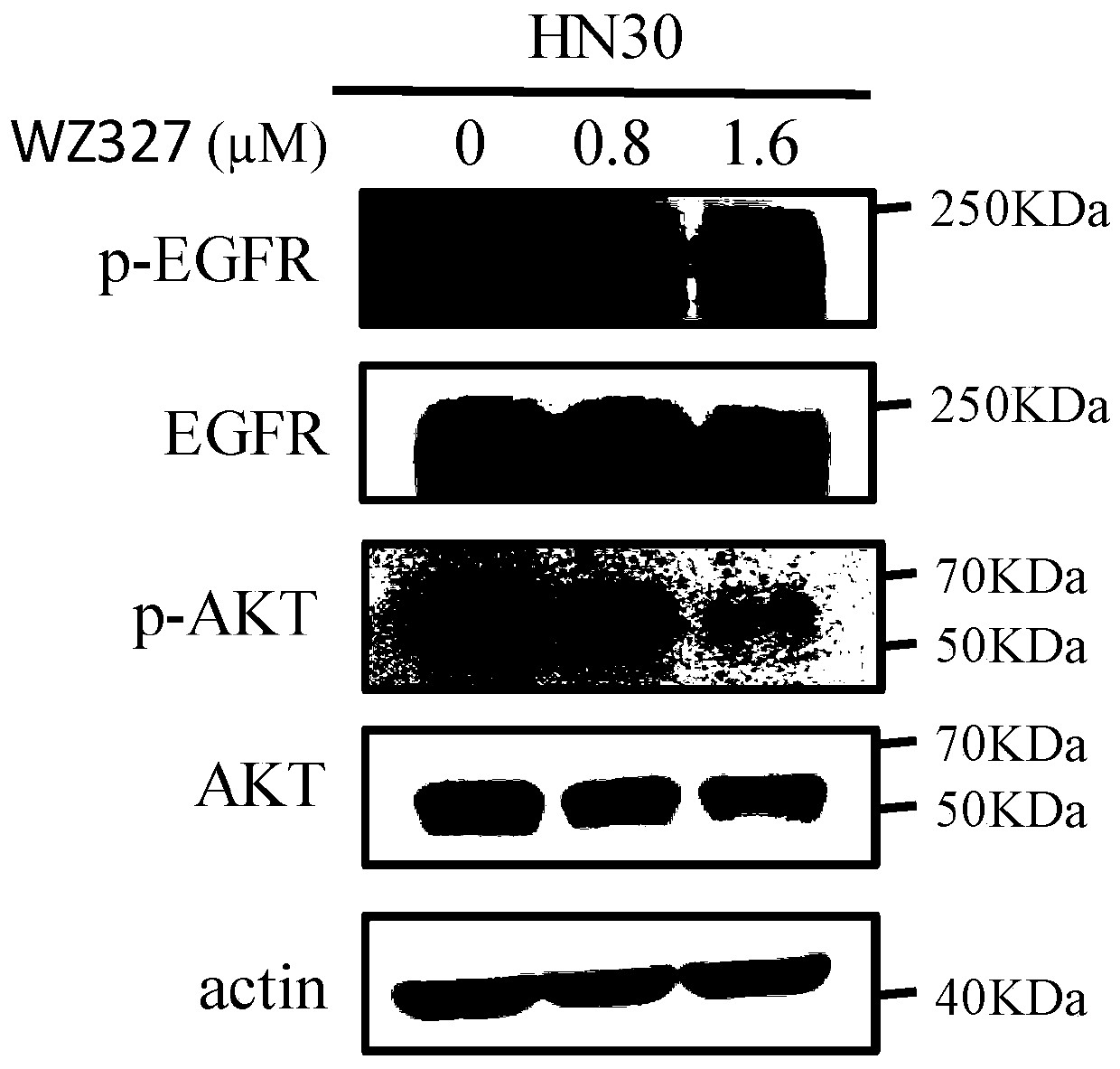
[0173] Example 2: The effect of compound WZ327 on EGFR phosphorylation and its downstream proteins in oral squamous cell carcinoma cell line HN30 detected by Western blotting
[0174] HN30 cells were treated with different concentrations of compound WZ327. After harvesting the cells, the protein was lysed and the protein concentration was determined. The samples were subjected to western blotting with 8% SDS-PAGE gel. The proteins on the gel were transfected to the NC membrane, and the membrane was stained with Ponceau. After the membrane was rinsed three times with PBS, it was blocked with 5% skimmed milk at room temperature for 1 hour, and the corresponding primary antibody and secondary antibody were applied successively, and developed by chemiluminescence.
[0175] The result is as figure 1 shown. Compound WZ327 can effectively inhibit EGFR phosphorylation in cells and inhibit downstream AKT phosphorylation. The results indicated that the compound WZ327 could effectiv...
Embodiment 3
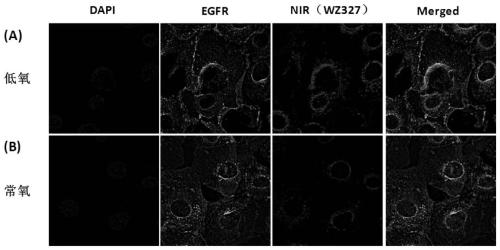
[0176] Example 3: Fluorescence imaging of compound WZ327 in oral squamous cell carcinoma cell line HN30
[0177] HN30 cells (3×10 5 / well) were inoculated in 6-well plates in advance (Fisherbrand coverslips were placed in the wells in advance), placed in normoxia and hypoxia (1% O 2 ) under the condition of 48h, blot dry medium, add medium containing 2μM compound WZ327 in the experimental well, place in normoxia and hypoxia (1% O 2 ) for 30 min, washed 3 times with PBS solution, fixed with 4% paraformaldehyde at room temperature for 10 min, washed 3 times with PBS solution, penetrated the membrane with 0.3% Txiton non-ionic detergent at room temperature for 5 min, washed 3 times with PBS solution, Immunofluorescence (DAPI and EGFR) staining, washed 3 times with PBS solution. Take out the glass slide, gently buckle it upside down on the slide, and seal the slide. Imaging was performed using a fluorescence microscope (IX83, Olympus, Japan) equipped with an infrared camera (Pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com