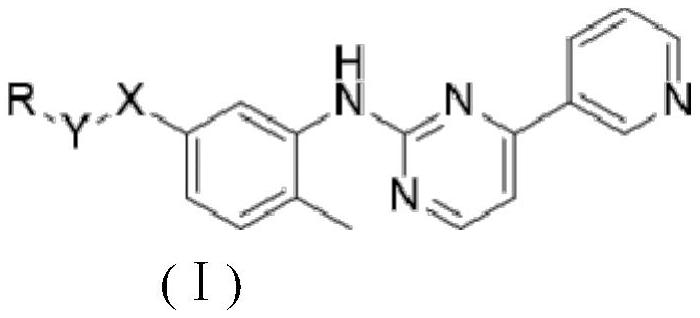
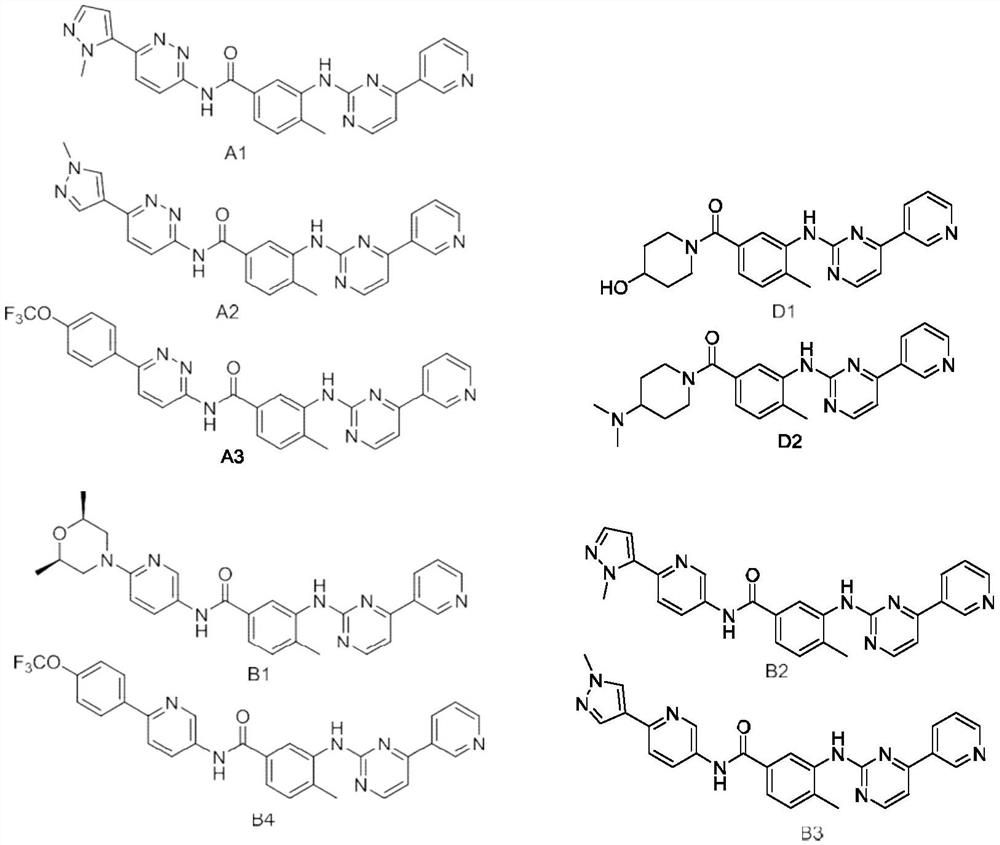
Smo/bcr-abl dual target inhibitor and its synthesis method and application
A technology of bcr-abl and synthesis method, which is applied in the field of Smo/Bcr-Abl dual-targeted inhibitor and its synthesis, and can solve the problems of complex interaction pharmacokinetics and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]
[0049] DMEDA: NN-isopropylethylamine; DCM: dichloromethane; DMF: N,N-dimethylformamide.
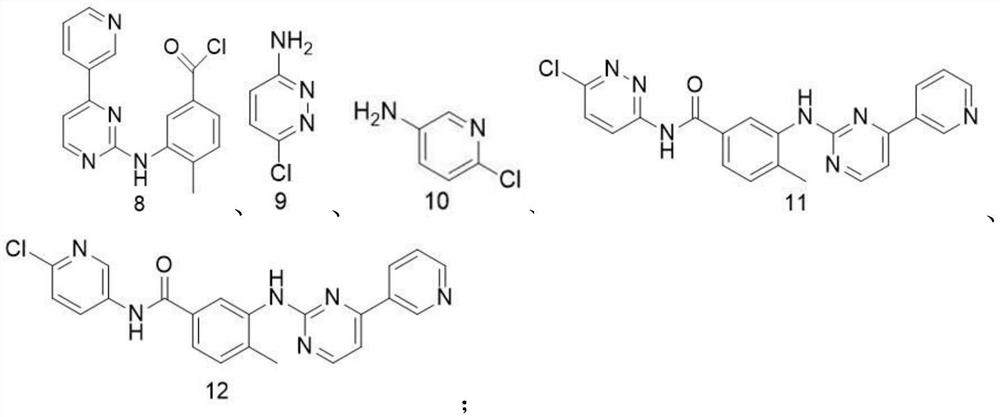
[0050] The synthesis of compound 8, its reaction process is as follows:
[0051] 1) Preparation of ethyl 3-bromo-4-methylbenzoate (compound 3):
[0052] Take the raw material 3-bromo-4-methylbenzoic acid (1.00g, 0.02mol), dissolve it in 25ml of absolute ethanol, slowly add 2.0ml of concentrated sulfuric acid with a concentration (mass fraction) of 98%, and react at reflux at 75°C for 5h . After the reaction was completed, the solvent was evaporated to dryness under reduced pressure, and the pH was adjusted to 7 with saturated aqueous sodium carbonate solution. A yellow oily substance was precipitated, extracted 3-4 times with dichloromethane, combined the organic phases, and washed with anhydrous NaSO 4 After drying, dichloromethane was distilled off under reduced pressure, and compound 3 (1.05 g, yield 93%) was obtained as a yellow oily liquid by column chromatography.
[...
Embodiment 2
[0072] Embodiment 2: Synthesis of A series of pyridazine derivatives, the reaction scheme is as follows:
[0073]
[0074] 1) Preparation of compound N-(6-chloropyridazin-3-yl)-4-methyl-3-[[4-(3-pyridyl)-2-pyrimidine]amino]benzamide (compound 11) :
[0075] The prepared 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidine]amino]benzoyl chloride was dissolved in 15ml of anhydrous dichloromethane, and the 3-amino-6-chloropyridazine (0.13g, 1.0mmol) was dissolved in 20ml of anhydrous dichloromethane, stirred at 0°C, and 2ml of N,N-diisopropylethylamine (DIPEA) and catalyst 4-dimethyl Pyridine (CAS number is 1122-58-3, the addition amount is 0.02g, 0.16mmol, DMAP), and then it is slowly added dropwise to 4-methyl-3-[[4-(3-pyridyl)-2- pyrimidine]amino]benzoyl chloride in dichloromethane. After the solution was added dropwise, it was placed at room temperature (25° C.) for overnight reaction. After the reaction, dilute with an appropriate amount of dichloromethane, wash with saturated bri...
Embodiment 3
[0096] Embodiment 3: Synthesis of B series pyridine derivatives:
[0097] 1) Compound N-(6-((2S,6R)-2,6-dimethylmorpholine)pyridin-3-yl)-4-methyl-3-((4-(pyridin-3-yl) The synthesis of pyrimidin-2-yl)amino)benzamide (compound B1) is shown below:
[0098]
[0099] Preparation of compound 14, namely (2S, 6R)-2,6-dimethyl-4-(5-nitropyridin-2-yl)morpholine:
[0100] Take 2-chloro-5-nitropyridine (1.73g, 0.01mol) and cis-2,6-dimethylmorpholine (1.15g, 0.01mol) in a round bottom flask, add 10ml of DMF to dissolve, and then Add K to it 2 CO 3 (2.76g, 0.02mol), react at 55°C for 6h. Cool to room temperature after the reaction, extract 3-4 times with ethyl acetate, combine organic phases, wash 2-3 times with water, anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure to obtain a crude product, which is purified by column chromatography to obtain compound 14 as a yellow solid (2.20 g, yield 93%).
[0101] The detection data of compound 14 are as follows:
[0102] MS ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com