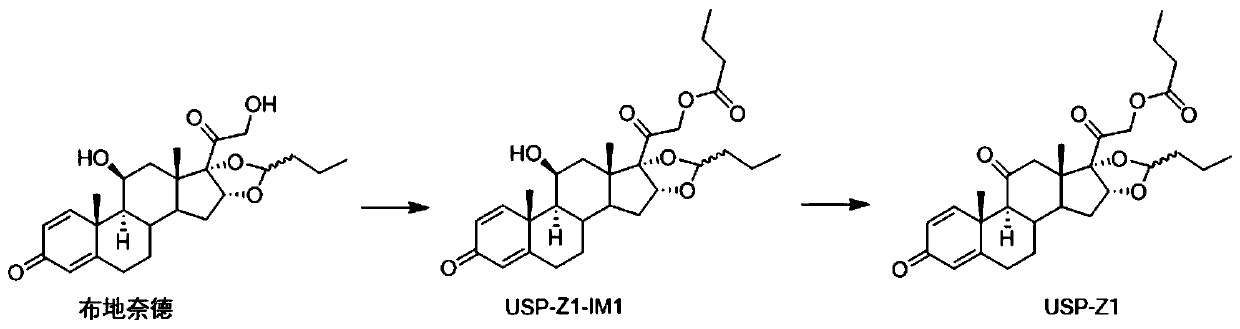
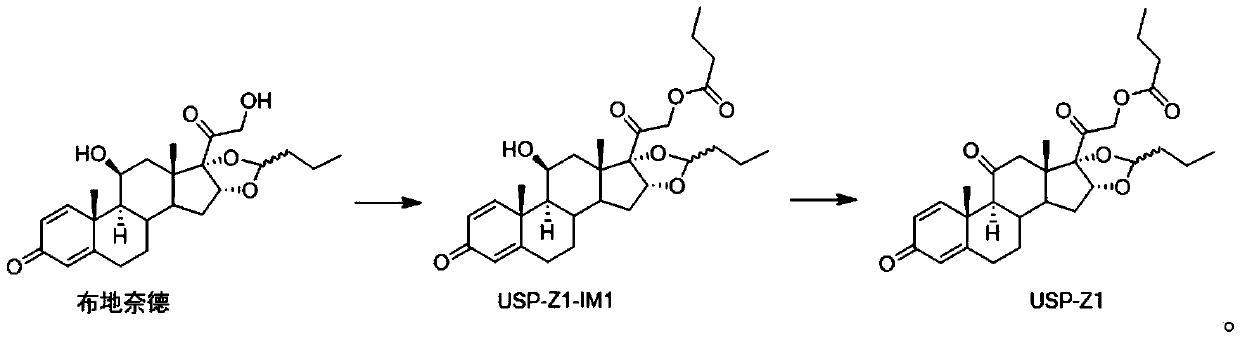
Synthesis method of budesonide impurity USP-Z1
A USP-Z1, USP-Z1-IM1 technology, applied in the field of drug impurity synthesis, to achieve the effect of simple process route, simple operation and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Step 1: Synthesis of compound USP-Z1-IM1
[0024]
[0025] Take 8.6g of budesonide in a 250mL three-neck bottle, add 80mL of 1,4-dioxane to dissolve, cool down to 0-10°C, add 6.06g of triethylamine as an acid-binding agent and 5.32g of butyryl chloride, control The temperature is not higher than 20°C, keep warm for 1-2 hours; after the reaction is completed, place the obtained reaction solution in 100mL water to quench the reaction, add 100mL dichloromethane for extraction, collect the organic phase and continue to dilute the water phase with 100mL dichloromethane Secondary extraction with methyl chloride, combining the organic phases obtained twice, successively using 50mL saturated sodium chloride solution and 50mL water to wash the organic phase, and evaporating the organic solvent under reduced pressure to obtain a foamy solid; adding 50mL of dichloromethane to the resulting foamy solid Dissolve, add petroleum ether at room temperature until cloudy, stop, crystal...
Embodiment 2
[0030] Step 1: Synthesis of compound USP-Z1-IM1
[0031]
[0032] Take 3.9g of budesonide in a 100mL three-necked bottle, add 40mL of 1,4-dioxane to dissolve, cool down to 0-10°C, add 2.94g of triethylamine as an acid-binding agent and 2.47g of butyric anhydride, control The temperature is not higher than 20°C, keep warm for 1-2 hours; after the reaction is completed, put the obtained reaction solution in 50mL water to quench the reaction, add 50mL dichloromethane to extract, collect the organic phase and continue to dilute the water phase with 50mL dichloromethane Chloromethane secondary extraction, combined two organic phases obtained, successively adopt 24mL saturated sodium chloride solution and 24mL water to wash the organic phase, evaporate the organic solvent under reduced pressure to obtain a foamy solid; add 25mL dichloromethane to the obtained foamy solid Dissolve, add petroleum ether at room temperature until cloudy, stop, crystallize at room temperature for 2 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com