Recombinant expression method for hypoglycemic polypeptide Aglycin and application thereof
An expression method and hypoglycemic technology, which is applied in the fields of genetic engineering and biopharmaceuticals, can solve the problems of cumbersome process and low efficiency of extraction and separation, and achieve the effect of easy aggregation, efficient acquisition, and small molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Construction of recombinant expression vector pET30a-Aglycin-Mxe-PT-ELK16
[0039] (1) According to the gene sequence of Aglycin, its DNA sequence Ag was synthesized at Gene Synthesis Company (Shanghai Sangong), and the following primers (Ag-F / Ag-R) were designed to amplify the Aglycin target gene:
[0040]
[0041] (2) Using the synthesized plasmid (pUC57-Ag) containing the Aglycin gene as a template, use the above amplification primers to amplify the Aglycin target gene. The specific steps are as follows:
[0042] a. PCR reaction system (50μL):
[0043]
[0044] b.PCR amplification reaction procedure:
[0045]
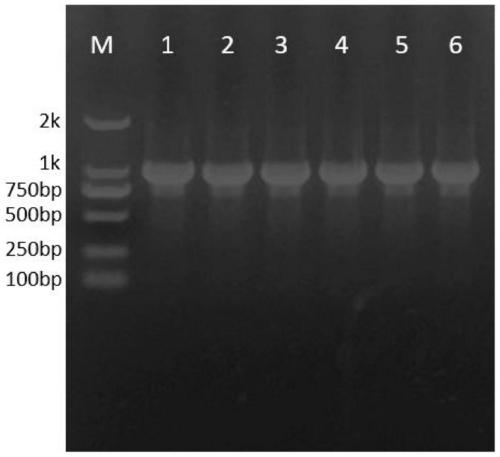
[0046] (3) After the PCR reaction finishes, carry out 1% (w / v) agarose gel electrophoresis identification, obtain the gene fragment that contains Aglycin about 165bp in size, purify and reclaim the PCR product, obtain the gene fragment that contains Aglycin, then Prepare a linear amplification reaction system, and insert the recovered Agl...
Embodiment 2
[0059] Example 2: Expression and optimization of Aglycin-Mxe-PT-ELK16 recombinant protein
[0060] (1) Transfer the recombinant expression vector pET30a-Aglycin-Mxe-PT-ELK16 constructed in Example 1 into Escherichia coli BL21(DE3) competent cells by chemical transformation, pick a single clone on the transformation plate and inoculate it into 6mL The LB liquid medium was cultured overnight at 37°C and 220 rpm as the seed solution.
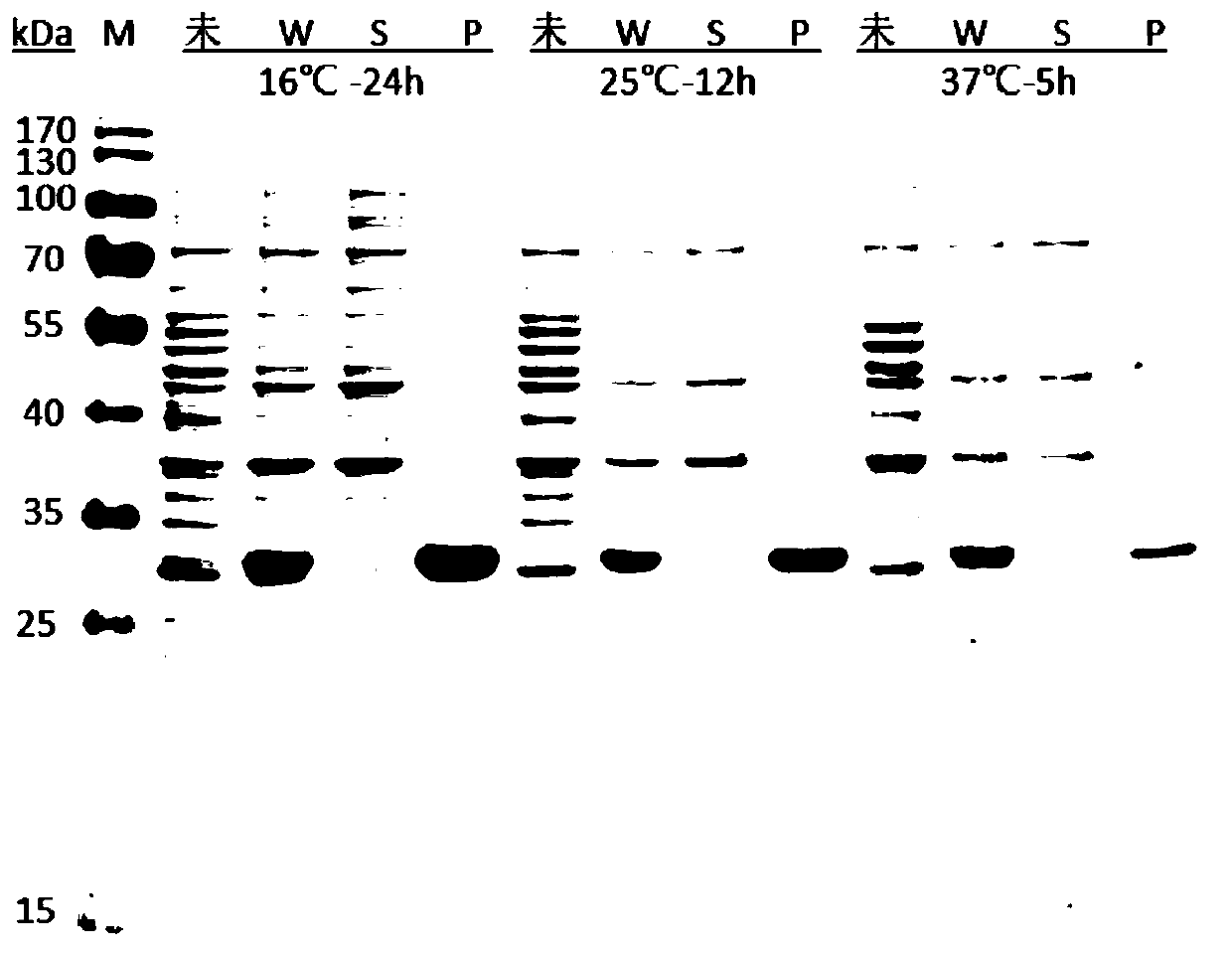
[0061] a. Temperature optimization of Aglycin-Mxe-PT-ELK16 expression: Inoculate the seed liquid into 6 test tubes containing 6 mL of fresh LB liquid medium at a ratio of 1:100 (v / v), place at 37°C, 220rpm cultured, when OD 600 When =0.6~0.8, 3 branches were added with 1mM IPTG for induction, and the other 3 branches were not added with inducer IPTG as a control, paired in pairs (1 branch was induced, 1 branch was not induced) to induce expression at 16°C, 25°C, and 37°C, respectively 24h, 12h, 5h.
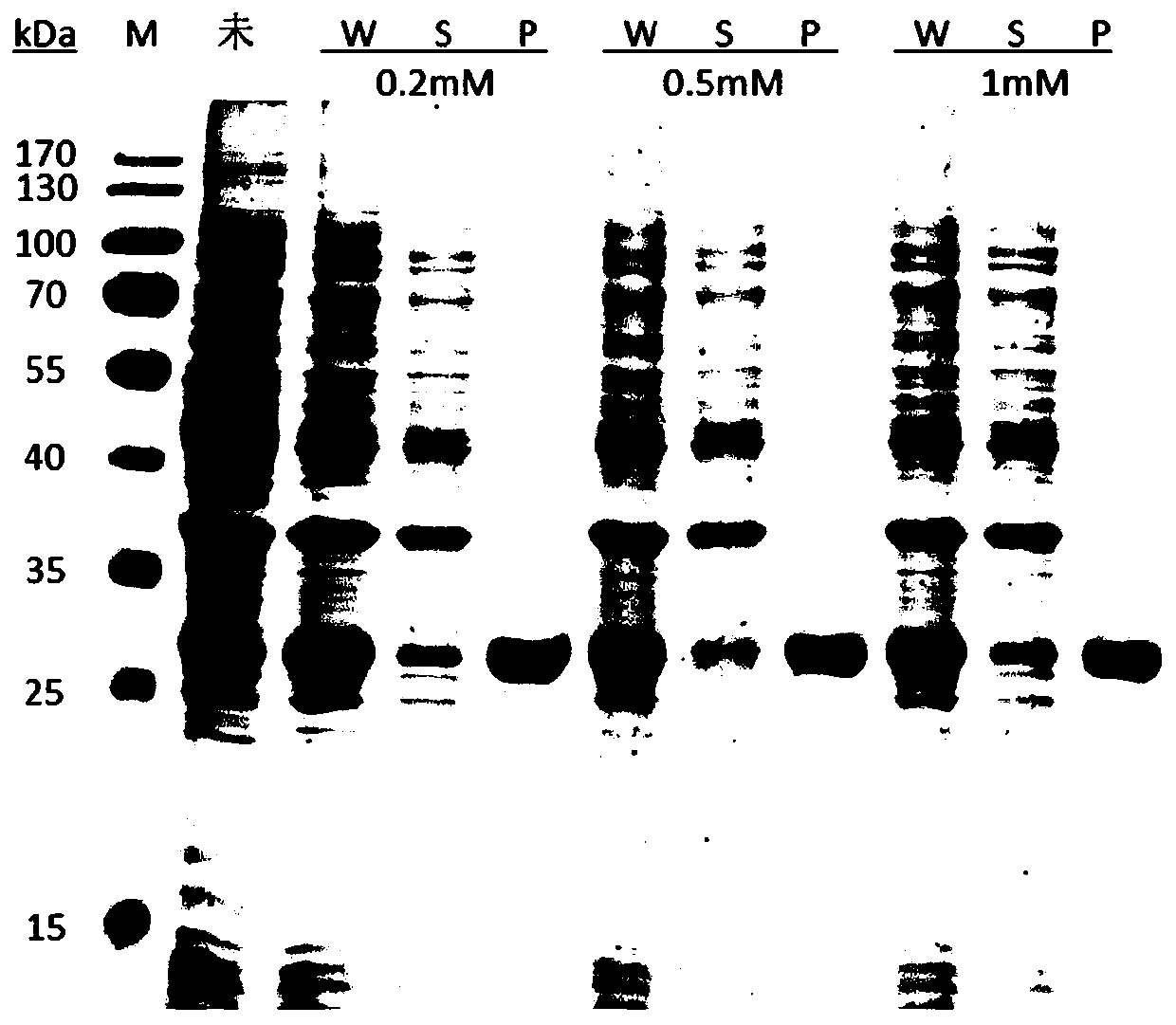
[0062] b. Optimizing the concentration of the...
Embodiment 3
[0068] Embodiment 3: DTT cleavage condition optimization of Aglycin-Mxe-PT-ELK16 protein
[0069] (1) Inoculate the single clone in Example 2 into 8 mL of LB liquid medium for overnight culture at 37° C. and 220 rpm as the seed solution. Inoculate the seed liquid into 600mL fresh LB liquid medium at a ratio of 1:100 (v / v), place it at 37°C, and cultivate it at 220rpm, when the OD 600 =0.6~0.8, add the inducer IPTG to a final concentration of 0.2mM, and then place in a shaker at 16°C to induce expression for 24h.
[0070] (2) After the expression, the cells were collected by centrifugation at 9000rpm for 10 min, resuspended in LysisBuffer, crushed by high pressure, and the precipitate was collected by centrifugation, and washed by adding washing buffer to the precipitate to obtain a precipitate sample.
[0071] a. Add the corresponding volume (20OD / mL) of cutting solution containing 40mM DTT to the precipitated samples, suspend evenly, place at 4°C for cutting, then take sampl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com