Method for rapidly dissolving nickel oxide, deacidifying nickel soap and removing impurities to produce nickel sulfate solution
A technology of nickel oxide and nickel sulfate, applied in the field of hydrometallurgy, can solve the problems of slow production energy consumption, slowed nickel dissolution rate, affecting the supply of nickel sulfate evaporation and crystallization solution, etc., and achieves the effect of rapid dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
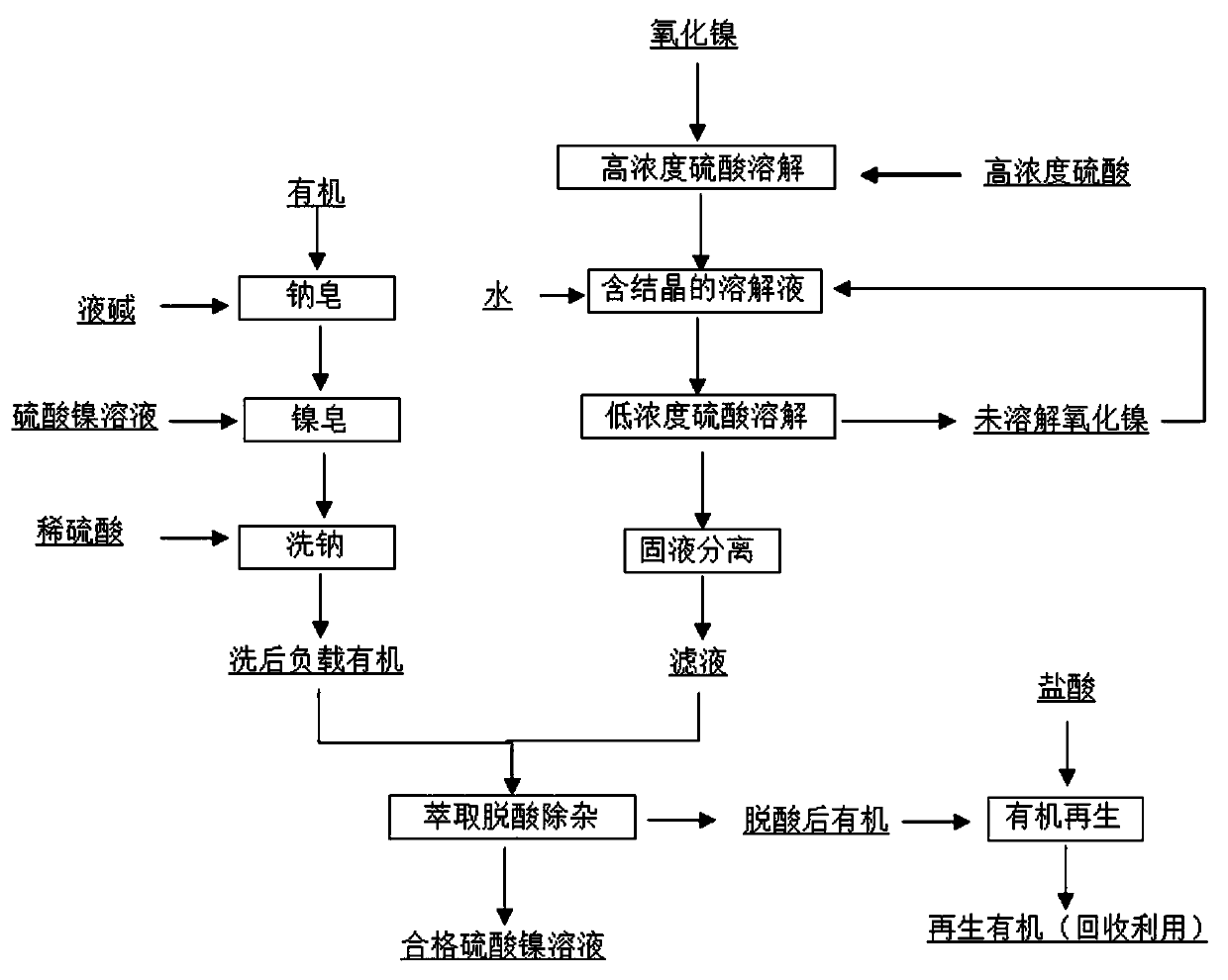
[0045] 1) Add sulfuric acid with a hydrogen ion concentration of 12mol / L to dissolved nickel oxide for dissolution reaction. The mass ratio of sulfuric acid volume to nickel oxide is 1:1, that is, 500mL of 12mol / L sulfuric acid, 500g of nickel oxide, and the reaction temperature is 90 ℃, reaction time 30min.
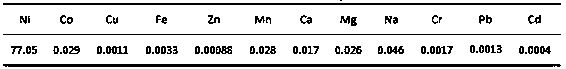
[0046](2) Then add water to dilute the nickel oxide solution containing crystals by 3 times and continue the reaction. Control the reaction temperature at 70°C, and the reaction time is 3 hours, and perform solid-liquid separation. After separation, the filtrate is obtained. The hydrogen ion concentration in the filtrate is 1.7mol / L, nickel ion 67.5g / L.
[0047] (3) Sodium soap: use P507 as the extractant, sulfonated kerosene as the diluent, mix sulfonated kerosene and P507 at a volume ratio of 4:1 (sulfonated kerosene volume 4L, P507 volume 1L) to obtain a mixed organic phase, Using liquid caustic soda with a mass fraction of 30% to carry out sodium soap on the mixed ...
Embodiment 2
[0056] 1) Add sulfuric acid with a hydrogen ion concentration of 14mol / L to nickel oxide for dissolution reaction. The mass ratio of sulfuric acid volume to nickel oxide is 1:2, that is, 500mL of 14mol / L sulfuric acid, 1000g of nickel oxide, and the reaction temperature is 95°C , The reaction time is 15min.
[0057] (2) Then add water to dilute the nickel oxide solution containing crystals by 4 times and continue the reaction. Control the reaction temperature at 80°C and the reaction time for 2 hours to perform solid-liquid separation. After separation, the filtrate is obtained. The hydrogen ion concentration in the filtrate is 1.49mol / L, nickel ion 60.5g / L.
[0058] (3) Sodium soap: use P507 as the extractant, sulfonated kerosene as the diluent, mix sulfonated kerosene and P507 at a volume ratio of 3:1 (sulfonated kerosene volume 3L, P507 volume 1L) to obtain a mixed organic phase, Use liquid caustic soda with a mass fraction of 30% to carry out sodium soap on the mixed org...
Embodiment 3
[0067] 1) Add sulfuric acid with a hydrogen ion concentration of 16mol / L to dissolved nickel oxide for dissolution reaction. The mass ratio of sulfuric acid volume to nickel oxide is 1:1.4, that is, 500mL of 16mol / L sulfuric acid, 700g of nickel oxide, and a reaction temperature of 92 ℃, reaction time 20min.
[0068] (2) Then add water to dilute the nickel oxide solution containing crystals by 3.5 times and continue the reaction. Control the reaction temperature at 90°C and the reaction time for 1.5 hours, and perform solid-liquid separation. After separation, the filtrate is obtained. The hydrogen ion concentration in the filtrate is 1.89mol / L, nickel ion 77.8g / L.
[0069] (3) Sodium soap: use P507 as the extractant, sulfonated kerosene as the diluent, mix sulfonated kerosene and P507 at a volume ratio of 3:2 (sulfonated kerosene volume 3L, P507 volume 2L) to obtain a mixed organic phase, Using liquid caustic soda with a mass fraction of 30% to carry out sodium soap on the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com