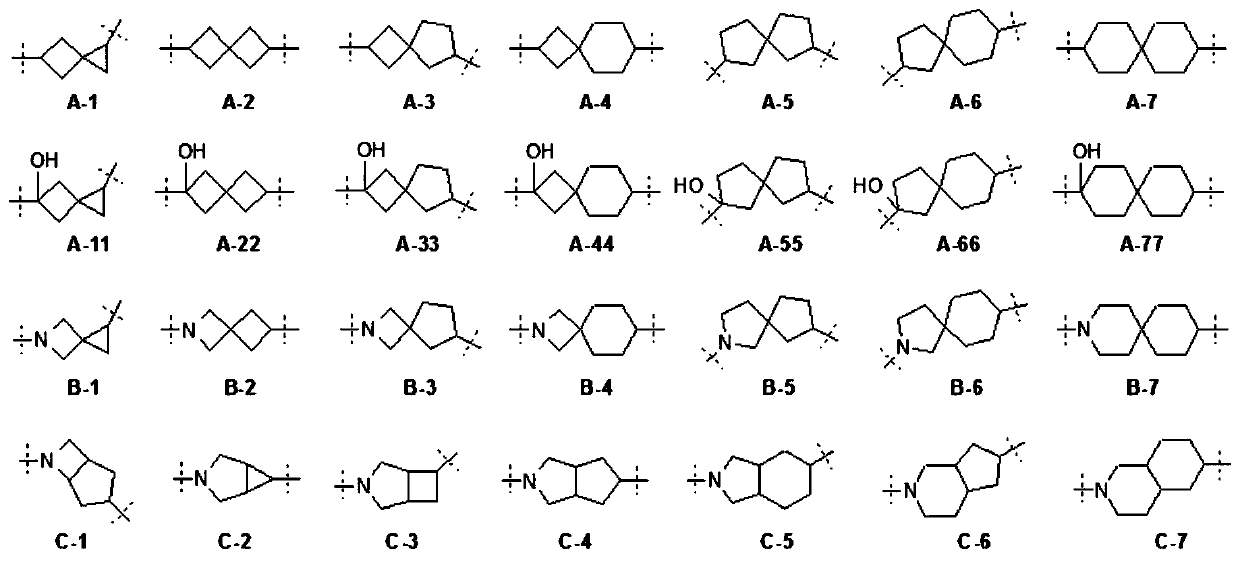
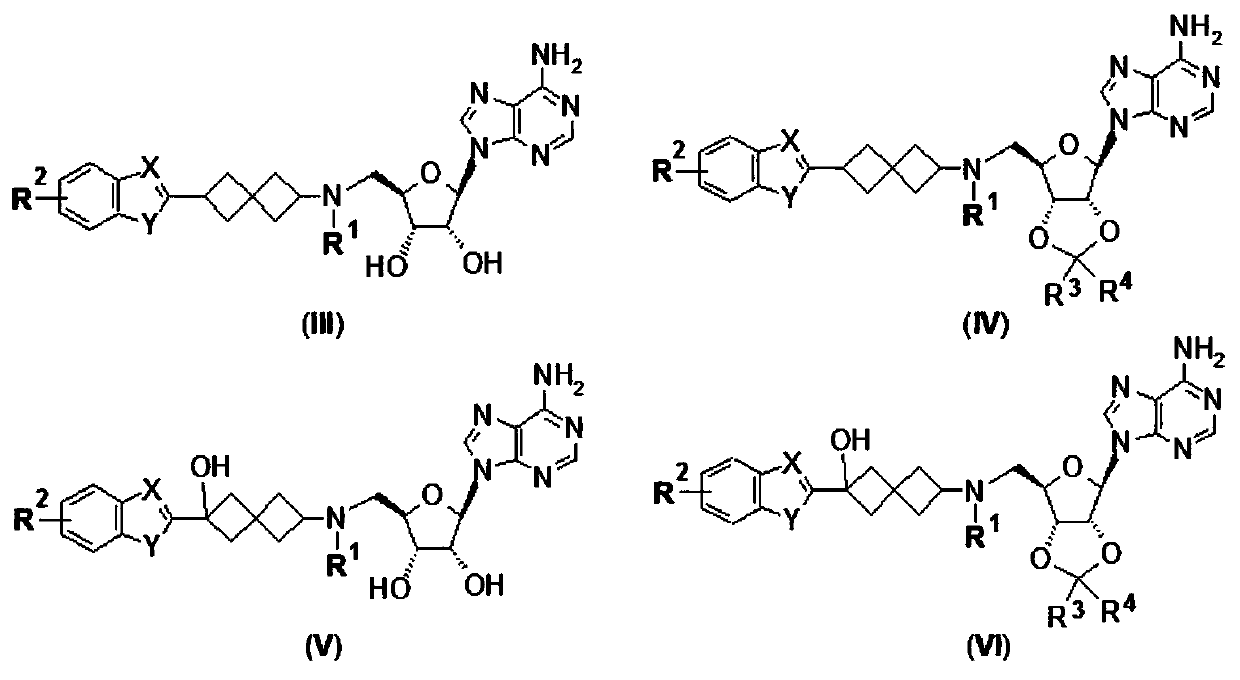
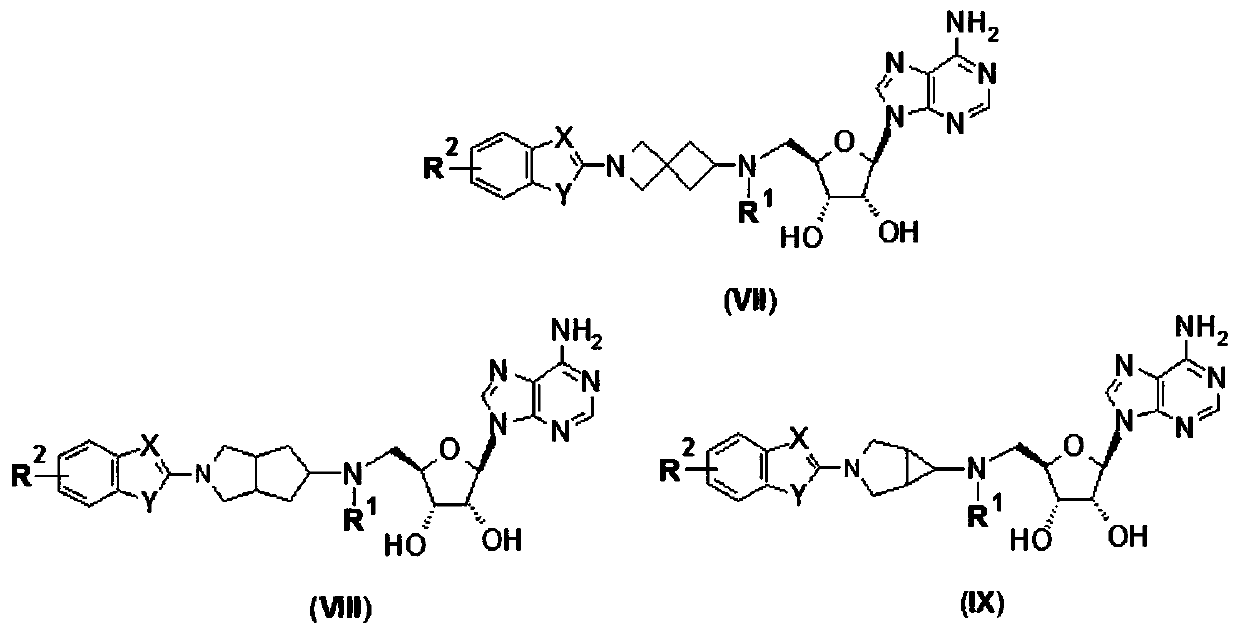
Purine compound containing bicyclic group, and preparation method thereof
A technology of compound and ring group, applied in the field of purine compound containing bicyclic group and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] 9-[(3aR,4R,6R,6aR)-6-([[6-(5-tert-butyl-1H-1,3-benzimidazol-2-yl)spiro[3.3]heptane-2- Base](propan-2-yl)amino]methyl)-2,2-dimethyl-tetrahydro-2H-furo[3,4-d][1,3]dioxolane-4 -yl]-9H-purin-6-amine (1):
[0093]
[0094] Its preparation reaction route is as follows Figure 10 As shown, the method is as follows:
[0095] The first step: [(3aR,4R,6R,6aR)-6-(6-amino-9H-purin-9-yl)-2,2-methyl-tetrahydro-2H-furo[3,4- d] Synthesis of [1,3]dioxolan-4-yl]methanol (I-2):
[0096] To (2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol (adenosine, I-1,50.0g, 187mmol) in acetone (1L) solution, add p-toluenesulfonic acid (96.6g, 561mmol) and triethyl orthoformate (83.1g, 561mmol) successively, the resulting solution was stirred at room temperature 14 hours, then basified to pH≈8 with saturated aqueous potassium carbonate solution. The reaction mixture was filtered and the filtrate was further concentrated in vacuo until a white solid appeared. The solid ...
Embodiment 2
[0109] Examples 2a / b, 2a and 2b
[0110] (2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-({[(2R,6R) / (2R,6S)-6-(5-tert-butyl Base-1H-1,3-benzimidazol-2-yl)spiro[3.3]heptane-2-yl](propan-2-yl)amino}methyl)oxolane-3,4-ol (2a / b), (2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-({[(2R,6R)-6-(5-tert-butyl Base-1H-1,3-benzimidazol-2-yl)spiro[3.3]heptane-2-yl](propan-2-yl)amino}methyl)oxolane-3,4-ol (2a) and (2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-({[(2R,6S)-6-(5-tert-butyl- 1H-1,3-benzimidazol-2-yl)spiro[3.3]heptane-2-yl](propan-2-yl)amino}methyl)oxolane-3,4-ol (2b ):
[0111]
[0112] Its preparation reaction scheme is as follows Figure 11 As shown, the method is as follows:
[0113] At 0°C, 9-[(3aR,4R,6R,6aR)-6-([[6-(5-tert-butyl-1H-1,3-benzimidazol-2-yl)spiro[3.3] Heptane-2-yl](propan-2-yl)amino]methyl)-2,2-dimethyl-tetrahydro-2H-furo[3,4-d][1,3]dioxa Cyclopentan-4-yl]-9H-purin-6-amine (Example 1, 700 mg, 1.14 mmol) was dissolved in 2.5 N hydrogen chloride in methanol (15 mL), a...
Embodiment 3
[0116] Examples 3a / b, 3a and 3b
[0117] (2R,3R,4S,5R)-2-(6-Amino-9H-purin-9-yl)-(S / R)-5-({[(2R,6R)-6-(5-tert-butyl Base-1H-1,3-benzimidazol-2-yl)spiro[3.3]heptane-2-yl](propan-2-yl)nitroso}methyl)oxolane-3,4 -alcohol (3a / b), (2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-(S)-5-({[(2R,6R)-6 -(5-tert-butyl-1H-1,3-benzimidazol-2-yl)spiro[3.3]heptane-2-yl](propan-2-yl)nitroso}methyl)oxetane Pentan-3,4-ol (3a) and (2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-(R)-5-({[(2R, 6R)-6-(5-tert-butyl-1H-1,3-benzimidazol-2-yl)spiro[3.3]heptane-2-yl](propan-2-yl)nitroso}methyl ) oxolane-3,4-ol (3b):
[0118]
[0119] Its preparation reaction scheme is as follows Figure 12 As shown, the method is as follows:
[0120] To (2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-({[(2R,6R)-6-(5-tert-butyl-1H-1 ,3-benzimidazol-2-yl)spiro[3.3]heptane-2-yl](propan-2-yl)amino}methyl)oxolane-3,4-ol (2a, 20mg, 0.03mmol) in dioxane / water (2mL / 0.6mL) solution was added 3-chloroperoxybenzoic acid (mCPBA, 6mg), the resu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com