Nickel zinc ferrite/bismuth tungstate magnetic composite photocatalytic material and preparation method thereof
A technology of composite photocatalysis and nickel zinc ferrite, which is applied in the direction of catalyst activation/preparation, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of low catalytic activity of visible light, tungstic acid Bismuth is difficult to recycle, etc., to achieve the effects of high visible light catalytic activity, wide visible light response range, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
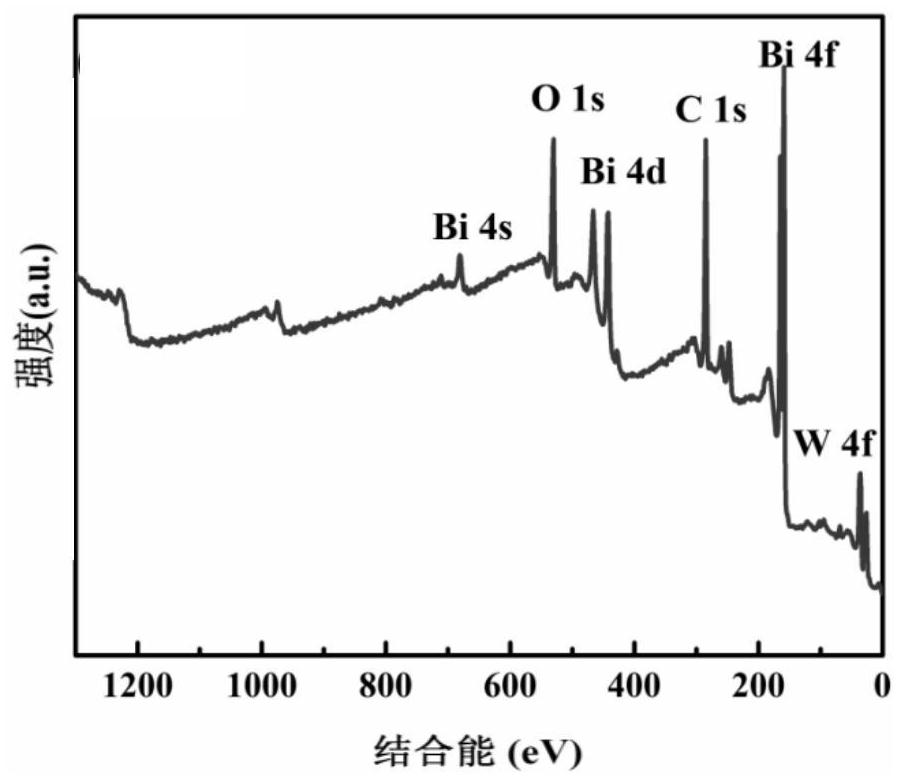
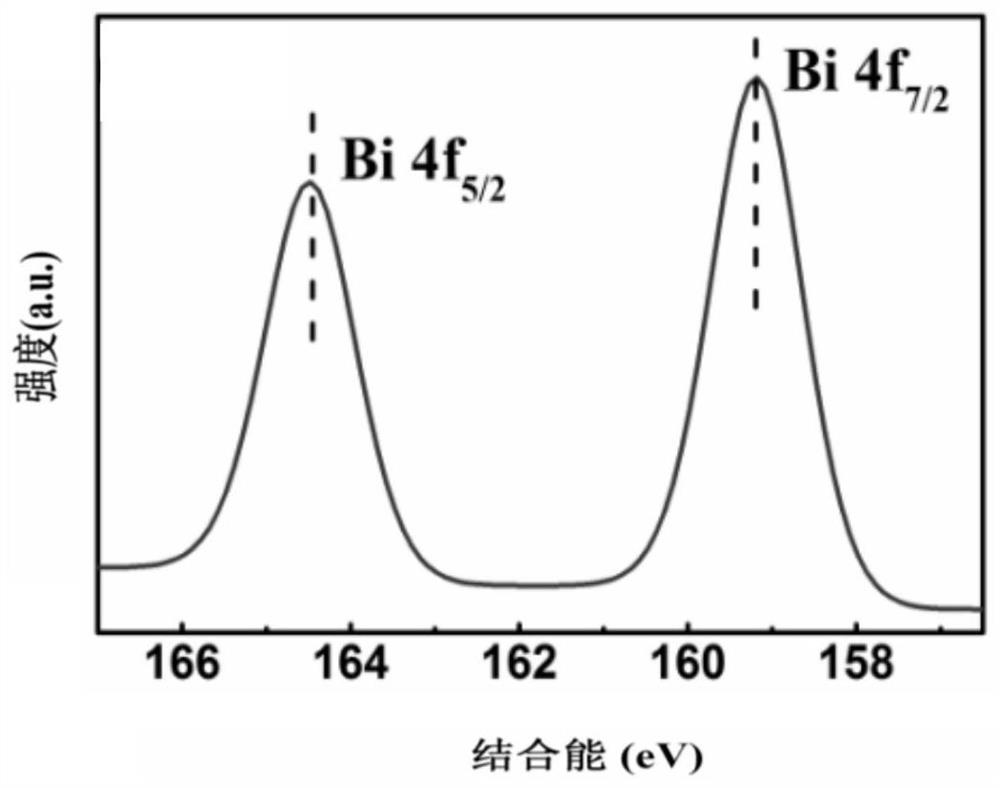
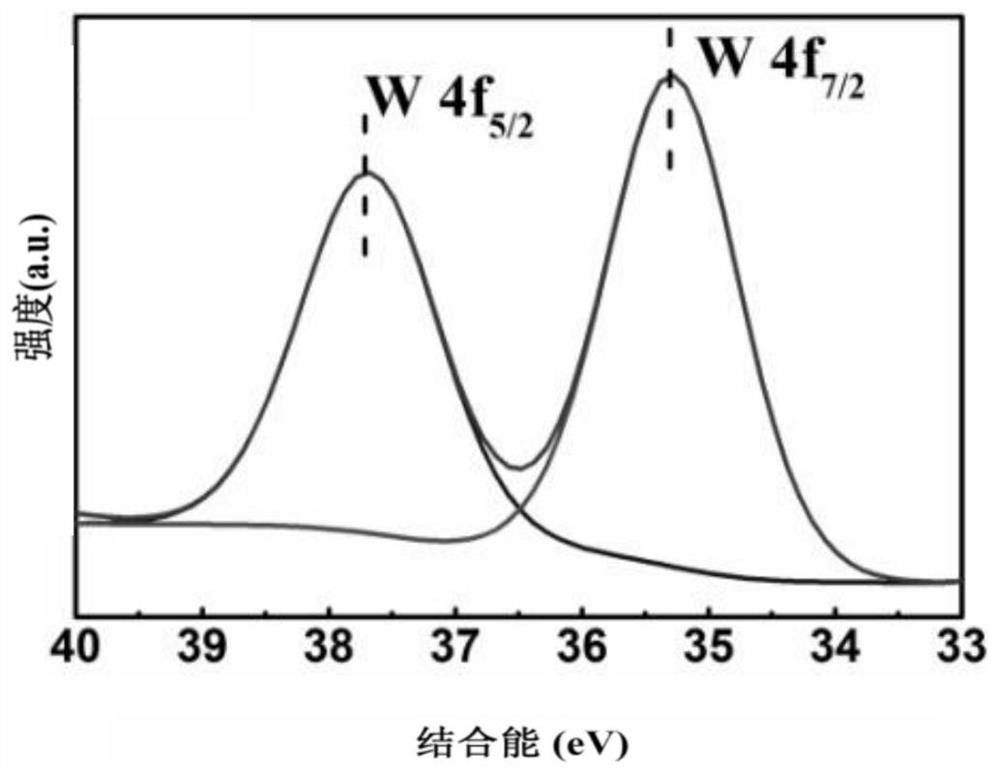
Image
Examples
Embodiment 1
[0057] Step 1: Take by weighing 0.1091g Ni(NO 3 ) 2 , 0.1115g Zn(NO 3 ) 2 and 0.6060g of Fe(NO 3 ) 3 , Dissolve it in a mixed liquid composed of 10mL water and 5mL liquid polyethylene glycol (PEG-400) and stir it rapidly for 120min, then add NH 3 ·H 2 O to adjust the pH to 10, continue stirring for 30 min, and transfer the resulting mixed liquid to a 50 mL stainless steel autoclave with a polytetrafluoroethylene liner for hydrothermal reaction at 180 °C for 5 h; Separation, the resulting precipitate was washed with water, washed with alcohol, dried in vacuum at 80°C for 7 hours, and ground to obtain Ni 0.5 Zn 0.5 Fe 2 o 4 , denoted as NZTY;
[0058] Step 2: Weigh 0.1050g of Ni 0.5 Zn 0.5 Fe 2 o 4 , it was added to 45mL of CTAB aqueous solution with a concentration of 8.23mmol / L and ultrasonically dispersed for 30min, and 2.9198g of Bi(NO 3 ) 3 ·5H 2 O, then add 2.5mL concentration of 4mol / L dilute nitric acid solution, high-speed magnetic stirring at room temp...
Embodiment 2
[0060] Step 1: Take by weighing 0.1091g Ni(NO 3 ) 2 , 0.1115g Zn(NO 3 ) 2 and 0.6060g of Fe(NO 3 ) 3 , Dissolve it in a mixed liquid composed of 10mL water and 5mL liquid polyethylene glycol (PEG-400) and stir it rapidly for 120min, then add NH 3 ·H 2 O to adjust the pH to 10, continue stirring for 30 min, and transfer the resulting mixed liquid to a 50 mL stainless steel autoclave with a polytetrafluoroethylene liner for hydrothermal reaction at 180 °C for 5 h; Separation, the resulting precipitate was washed with water, washed with alcohol, dried in vacuum at 80°C for 7 hours, and ground to obtain Ni 0.5 Zn 0.5 Fe 2 o 4 , denoted as NZTY;
[0061] Step 2: Weigh 0.1050g of Ni 0.5 Zn 0.5 Fe 2 o 4 , was added to 45 mL of CTAB aqueous solution with a concentration of 8.23 mmol / L and ultrasonically dispersed for 30 min, and 1.4599 g of Bi(NO 3 ) 3 ·5H 2 O, then add 2.5mL concentration of 4mol / L dilute nitric acid solution, high-speed magnetic stirring at room t...
Embodiment 3
[0063] Step 1: Take by weighing 0.1091g Ni(NO 3 ) 2 , 0.1115g Zn(NO 3 ) 2 and 0.6060g of Fe(NO 3 ) 3 , Dissolve it in a mixed liquid composed of 10mL water and 5mL liquid polyethylene glycol (PEG-400) and stir it rapidly for 120min, then add NH 3 ·H 2 O to adjust the pH to 10, continue stirring for 30 min, and transfer the resulting mixed liquid to a 50 mL stainless steel autoclave with a polytetrafluoroethylene liner for hydrothermal reaction at 180 °C for 5 h; Separation, the resulting precipitate was washed with water, washed with alcohol, dried in vacuum at 80°C for 7 hours, and ground to obtain Ni 0.5 Zn 0.5 Fe 2 o 4 , denoted as NZTY;
[0064] Step 2: Weigh 0.1050g of Ni 0.5 Zn 0.5 Fe 2 o 4 , it was added to 45mL of CTAB aqueous solution with a concentration of 8.23mmol / L and ultrasonically dispersed for 30min, adding 0.9733gBi(NO 3 ) 3 ·5H 2 O, then add 2.5mL concentration of 4mol / L dilute nitric acid solution, high-speed magnetic stirring at room tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com