Method for preparing high-purity naphthalic acid by taking beta-methylnaphthalene as raw material
A technology of naphthalene dicarboxylic acid and methylnaphthalene, which is applied in the field of coal chemical industry, can solve the problems of difficulty in obtaining raw materials and high cost, and achieve the effect of abundant sources, mild reaction conditions and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
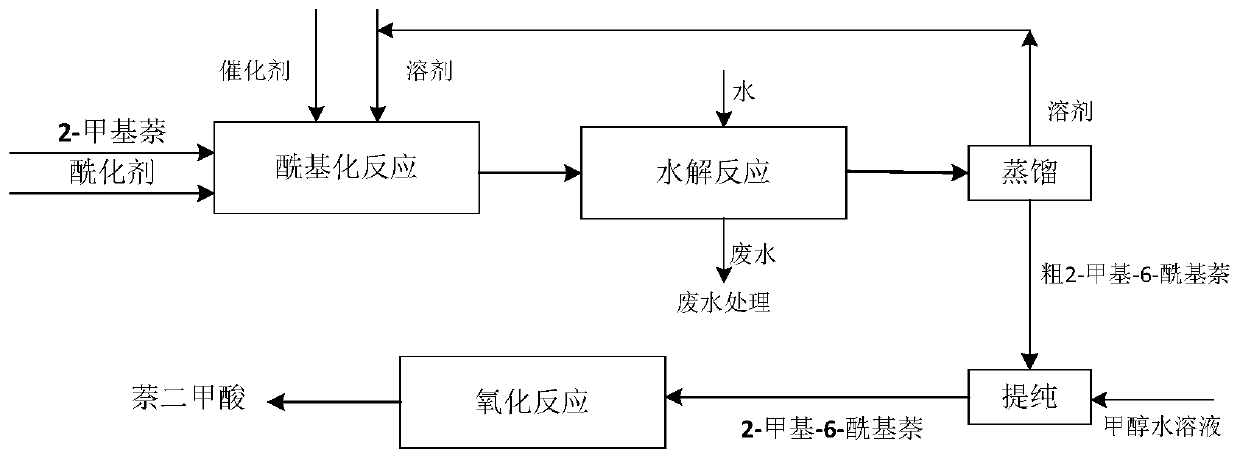
[0033] Put the stirred reactor into a low-temperature tank, add 300g of nitrobenzene, then add 50g of anhydrous aluminum trichloride, and finally add 26g of propionyl chloride, control the temperature at 0°C, and react for 10 minutes to prepare an acylation solution. Add 300g of nitrobenzene in a stirred reactor, and then add 30g of 2-methylnaphthalene to prepare a raw material solution. The two materials were mixed and reacted in a stirring reactor, the reaction time was 120min, and the reaction temperature was controlled at 35°C. The reacted products enter the hydrolysis reactor, and the reaction temperature is controlled at 40°C. After washing with water, distill under reduced pressure to obtain a crude product, which is then recrystallized with methanol containing 20% water. Finally, 2-methyl-6-propionylnaphthalene with a yield of 91% and a purity of 98.5% can be obtained.
[0034] Add 2-methyl-6-propionylnaphthalene to the gas-liquid stirred bubbling reactor, add 100g...
Embodiment 2
[0036] Put the stirred reactor into a low-temperature tank, add 200g of nitrobenzene, then add 35g of anhydrous aluminum trichloride, and finally add 20g of propionyl chloride, control the temperature at 5°C, and react for 15 minutes to prepare an acylation solution. Add 200g of nitrobenzene in a stirred reactor, and then add 22g of 2-methylnaphthalene to prepare a raw material solution. The two materials were mixed and reacted in a stirring reactor, the reaction time was 180min, and the reaction temperature was controlled at 40°C. The reacted products enter the hydrolysis reactor, and the reaction temperature is controlled at 50°C. After washing with water, distill under reduced pressure to obtain a crude product, which is then recrystallized with methanol containing 10% water. Finally, 2-methyl-6-propionylnaphthalene with a yield of 90% and a purity of 98.6% can be obtained.
[0037] Add 2-methyl-6-propionylnaphthalene to the gas-liquid stirred bubbling reactor, add 80g gl...
Embodiment 3
[0039] Put the stirred reactor into a low-temperature tank, add 100g of nitrobenzene, then add 20g of anhydrous aluminum trichloride, and finally add 9g of propionyl chloride, control the temperature at 0°C, and react for 30 minutes to prepare an acylation solution. Add 100 g of nitrobenzene into a stirred reactor, and then add 10 g of 2-methylnaphthalene to prepare a raw material solution. The two materials were mixed and reacted in a stirring reactor, the reaction time was 60min, and the reaction temperature was controlled at 25°C. The reacted products enter the hydrolysis reactor, and the reaction temperature is controlled at 30°C. After washing with water, distill under reduced pressure to obtain a crude product, and then recrystallize with methanol containing 30% water. Finally, 2-methyl-6-propionylnaphthalene with a yield of 90% and a purity of 98.8% can be obtained.
[0040] Add 2-methyl-6-propionylnaphthalene to the gas-liquid stirred bubbling reactor, add 70g glacia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com