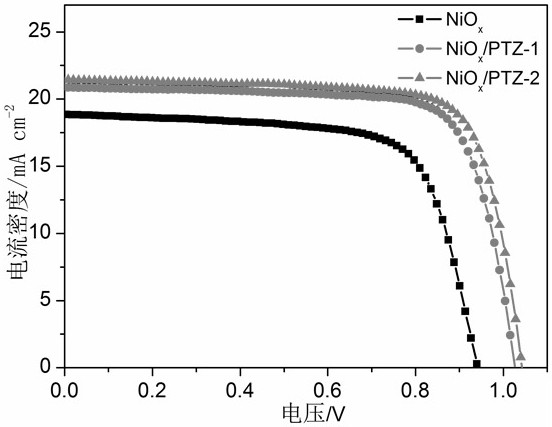
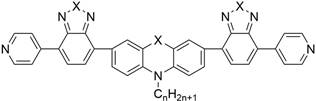
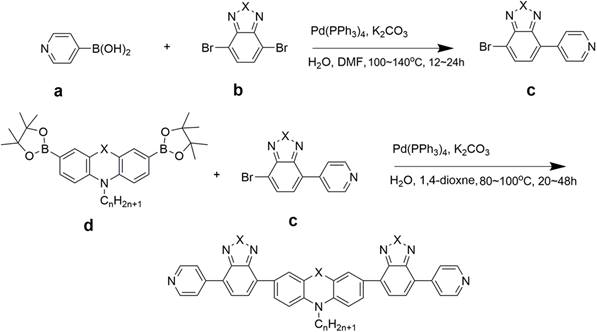
A kind of conjugated organic small molecule containing double-terminal pyridine and its preparation method and application
A technology of terminal pyridine and small molecules, which is applied in the field of solar cells, can solve the problems of reducing battery performance, affecting the morphology and compactness of perovskite light-absorbing layers, and poor contact, so as to achieve hysteresis suppression and energy conversion efficiency and open-circuit photovoltage increase, improve the effect of short-circuit photocurrent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: When X=S, m=0, n=6, the preparation of conjugated small organic molecules (referred to as PTZ-1)
[0041] Prepared according to the literature method [M.Sailer, M.Nonnenmacher, T.Oeser and T.J.J.Müller.Eur.J.Org.Chem.2006,423–435], specifically: using 1,4-dioxane as a solvent, Compound e (X=S, m=0) and bisboronic acid substituted phenothiazine d (X=S, n=6) were mixed at a molar ratio of 3:1, and Pd(PPh 3 ) 4 Reaction with potassium carbonate and water at 80°C for 24 hours, and the product PTZ-1 was obtained as a yellow solid through column chromatography with a yield of 78%. 1 H NMR 8.59(d, J=4.8Hz, 4H), 7.43~7.45(m, 8H), 6.94(d, J=8.4Hz, 2H), 3.89(t, J=7.2Hz, 2H), 1.83(m ,2H),1.47(m,2H),1.32(m,4H),0.89(t,J=6.4Hz,3H). 13 C NMR (100MHz, DMSO-d 6 ) 149.9, 147.0, 145.5, 132.0, 128.4, 125.5, 124.7, 120.7, 115.7, 47.7, 31.3, 26.6, 26.5, 22.5, 13.9. ESI-HRMS (m / z): Calcd.for C 28 h 27 N 3 S: 438.1998. Found: 438.1998.
Embodiment 2
[0042] Example 2: When X=S, m=1, and n=6, the preparation of conjugated small organic molecules (referred to as PTZ-2):
[0043] Specifically: using 1,4-dioxane as a solvent, compound e (X=S, m=1) and bisboronic acid-substituted phenothiazine d (X=S, n=6) in a molar ratio of 2.5: 1 mix. Add Pd(PPh 3 )4 React with potassium carbonate and water at 80°C for 24h, and get the product PTZ-2 through column chromatography with a yield of 68%. 1 H NMR (400MHz, DMSO-d 6 )8.78(d, J=6.0Hz, 4H), 7.94~7.95(m, 4H), 7.86~7.89(m, 4H), 7.78~7.82(m, 4H), 7.06(d, J=8.8Hz, 2H ),3.99(t,J=7.2Hz,2H),1.94(m,2H),1.52(m,2H),1.38(m,4H),0.92(t,J=7Hz,3H). 13 C NMR (100MHz, DMSO-d 6 ) 150.1, 144.6, 133.7, 131.3, 129.6, 128.8, 128.5, 127.9, 126.7, 124.4, 123.5, 115.3, 47.8, 31.5, 26.8, 26.7, 22.6, 14.0. ESI-HRMS (m / z): Calcd.for C 40 h 31 N 7 S 3 :706.1896. Found: 706.1876.
Embodiment 3
[0044] Embodiment 3: When X=S, m=0, n=1, the preparation of conjugated small organic molecules (referred to as PTZ-3)
[0045] Prepared according to the literature method [M.Sailer, M.Nonnenmacher, T.Oeser and T.J.J.Müller.Eur.J.Org.Chem.2006,423–435], specifically: using 1,4-dioxane as a solvent, Compound e (X=S, m=0) and bisboronic acid substituted phenothiazine d (X=S, n=1) were mixed at a molar ratio of 3:1, and Pd(PPh 3 ) 4 Reaction with potassium carbonate and water at 85°C for 20 h, the product PTZ-3 was obtained as a yellow solid through column chromatography, and the yield was 76%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com