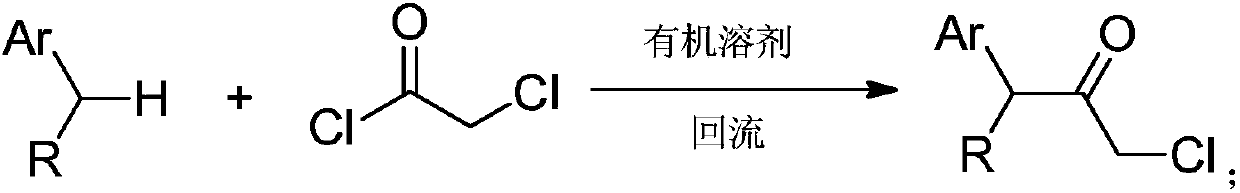
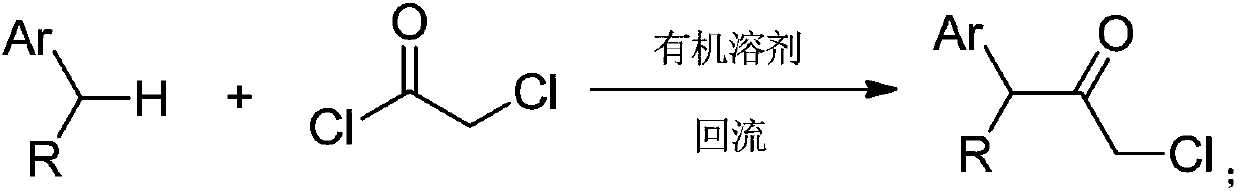
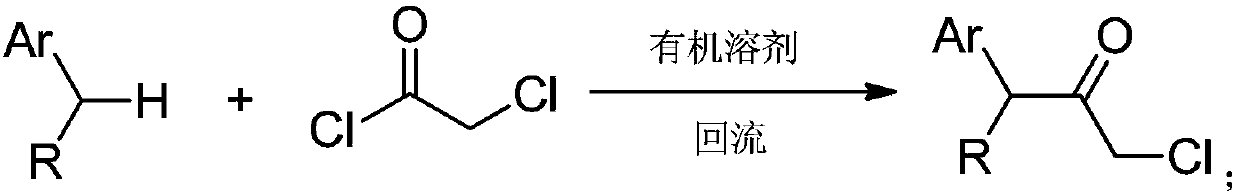
Synthesizing method for chloroacetamide compound
A technology of chloroacetamide and synthesis method, which is applied to the preparation of organic compounds, chemical instruments and methods, preparation of carboxylic acid amides, etc. Equipment requirements, the effect of high product yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0045] A kind of synthetic method of chloroacetamide compound, in reaction kettle, 107.16kg secondary amine compound A1 is dissolved in 53.58kg benzene, is warmed up to reflux, 112.94kg chloroacetyl chloride is added thereto, reflux reaction 0.5 hour, removes Solvent benzene, obtain 179kg chloroacetamide compound B1, productive rate is 97%, purity 98.4%; And reactor is connected with the water absorption device of hydrogen chloride gas, the hydrogen chloride gas produced in the reaction is absorbed by water absorption device, obtains industrial hydrochloric acid.
Embodiment 2
[0047]
[0048] A kind of synthetic method of chloroacetamide compound alachlor, in reaction kettle, 193.29kg secondary amine compound A2 is dissolved in 9664.5kg organic solvent toluene, is heated up to reflux, 338.82kg chloroacetyl chloride is added thereinto, reflux reaction 20 Hour, obtain 251kg chloroacetamide compound B2 (alachlor), productive rate is 93%, purity 98.2%; Reactor is connected with the water absorber of hydrogen chloride gas, and the hydrogen chloride gas produced in the reaction is absorbed by water absorber, Get industrial hydrochloric acid.
Embodiment 3
[0050]
[0051] A kind of synthetic method of chloroacetamide compound acetochlor, in reaction kettle, 193.29kg secondary amine compound A3 is dissolved in 386.58kg organic solvent sherwood oil, heat up to reflux, add 225.88kg chloroacetyl chloride wherein, reflux reaction 5 hours, add water 70kg and carry out washing, leave standstill for half an hour to separate liquid, organic phase removes solvent, obtains 246 chloroacetamide compounds B3 (acetochlor), productive rate is 91%, purity 98.1%; Reactor is connected with A water absorption device for hydrogen chloride gas, the hydrogen chloride gas generated in the reaction is absorbed by the ethanol aqueous solution with a mass concentration of 0.5% in the water absorption device to obtain industrial hydrochloric acid.
[0052] Inside the water absorption device is; the temperature inside the water absorption device is 0-20°C
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com