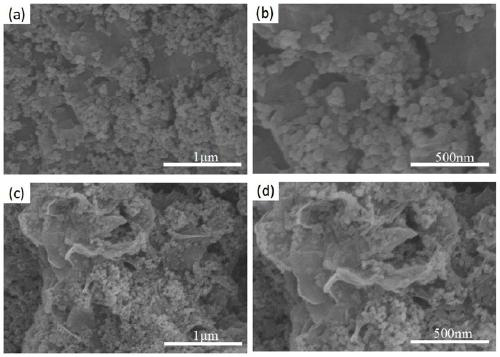
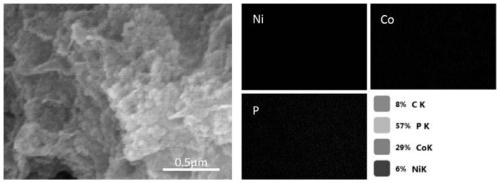
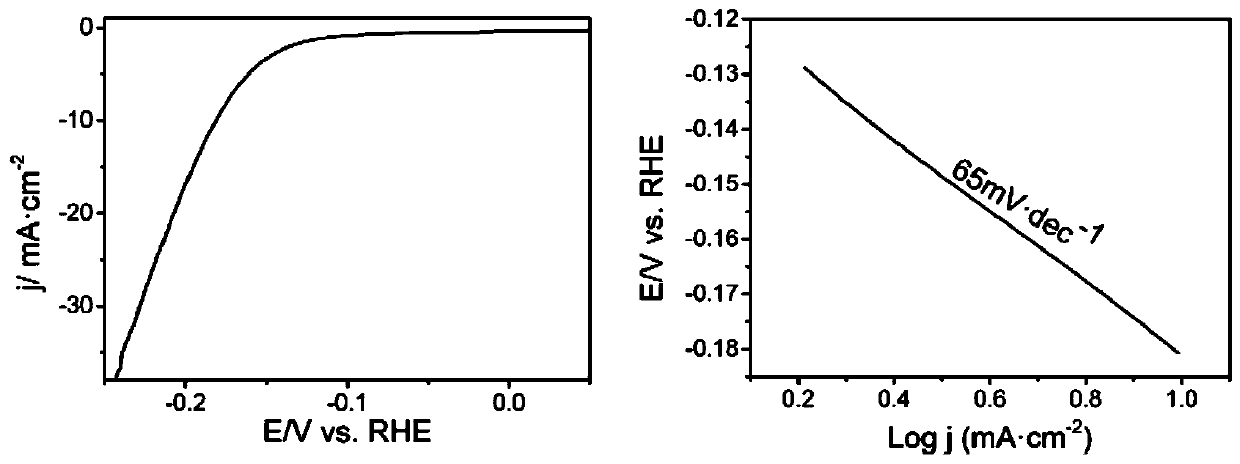
Preparation method of bimetallic phosphide composite reduction graphene nano electrocatalytic material
An electrocatalytic material, graphene technology, applied in the field of material chemistry, can solve problems such as poor conductivity, unstable structure, and obstruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Preparation of NiCo-MOF74 / GO nanocomposites:
[0025] (a) adding 25 mg of single-layer graphene oxide to 25 mL of deionized water, ultrasonicating for 4 hours until the solution is uniform, and obtaining a graphene oxide solution with a concentration of 1 mg / mL;
[0026] (b) 1.71mmol cobalt acetate tetrahydrate and 0.29mmol nickel acetate tetrahydrate were added respectively to the graphene oxide solution prepared by step (a) and stirred for 30min until the solution was uniform to obtain solution A;
[0027] (c) Add 1 mmol of 2,5-dihydroxyterephthalic acid and 4 mmol of sodium hydroxide into 20 mL of deionized water, and stir for 30 min to obtain solution B;
[0028] (d) stirring and reacting solution A and solution B at room temperature for 24h;
[0029] (e) The product was centrifuged, washed three times with water and ethanol, and then dried in a freeze dryer at -80°C for 24 hours.
[0030] (2) Preparation of NiCo / P-rGO material:
[0031] The product bimetalli...
Embodiment 2
[0034] (1) Preparation of NiCo-MOF74 / GO nanocomposites:
[0035] (a) adding 25 mg of single-layer graphene oxide to 50 mL of deionized water, ultrasonicating for 4 hours until the solution is uniform, and obtaining a graphene oxide solution with a concentration of 0.5 mg / mL;
[0036] (b) 1.71mmol cobalt acetate tetrahydrate and 0.29mmol nickel acetate tetrahydrate were added respectively to the graphene oxide solution prepared by step (a) and stirred for 30min until the solution was uniform to obtain solution A;
[0037] (c) 1 mmol of 2,5-dihydroxyterephthalic acid and 4 mmol of sodium hydroxide were added to 30 mL of deionized water, and stirred for 30 min to obtain solution B;
[0038] (d) stirring and reacting solution A and solution B at room temperature for 18h;
[0039] (e) The product was centrifuged, washed four times with water and ethanol, and then dried in a freeze dryer at -80° C. for 30 h.
[0040] (2) Preparation of NiCo / P-rGO material:
[0041]The product bim...
Embodiment 3
[0043] (1) Preparation of NiCo-MOF74 / GO nanocomposites:
[0044] (a) adding 25 mg of single-layer graphene oxide to 40 mL of deionized water, ultrasonicating for 4 hours until the solution is uniform, and obtaining a graphene oxide solution with a concentration of 0.625 mg / mL;
[0045] (b) 1.71mmol cobalt acetate tetrahydrate and 0.29mmol nickel acetate tetrahydrate were added respectively to the graphene oxide solution prepared by step (a) and stirred for 30min until the solution was uniform to obtain solution A;
[0046] (c) Add 1 mmol of 2,5-dihydroxyterephthalic acid and 4 mmol of sodium hydroxide into 20 mL of deionized water, and stir for 30 min to obtain solution B;
[0047] (d) stirring and reacting solution A and solution B at room temperature for 36h;
[0048] (e) The product was centrifuged, washed 5 times with water and ethanol, and then dried in a freeze dryer at -80°C for 36 hours.
[0049] (2) Preparation of NiCo / P-rGO material:
[0050] The product bimetalli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com