1-phenyl-4-penten-1-one derivative and its synthesis method and application
A synthesis method and derivative technology, applied in the field of 1-phenyl-4-penten-1-one derivatives and their synthesis, can solve the problems of severe reaction conditions, cumbersome operation, difficult selectivity control, etc., and achieve high Effects of regioselectivity, high step economy, and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
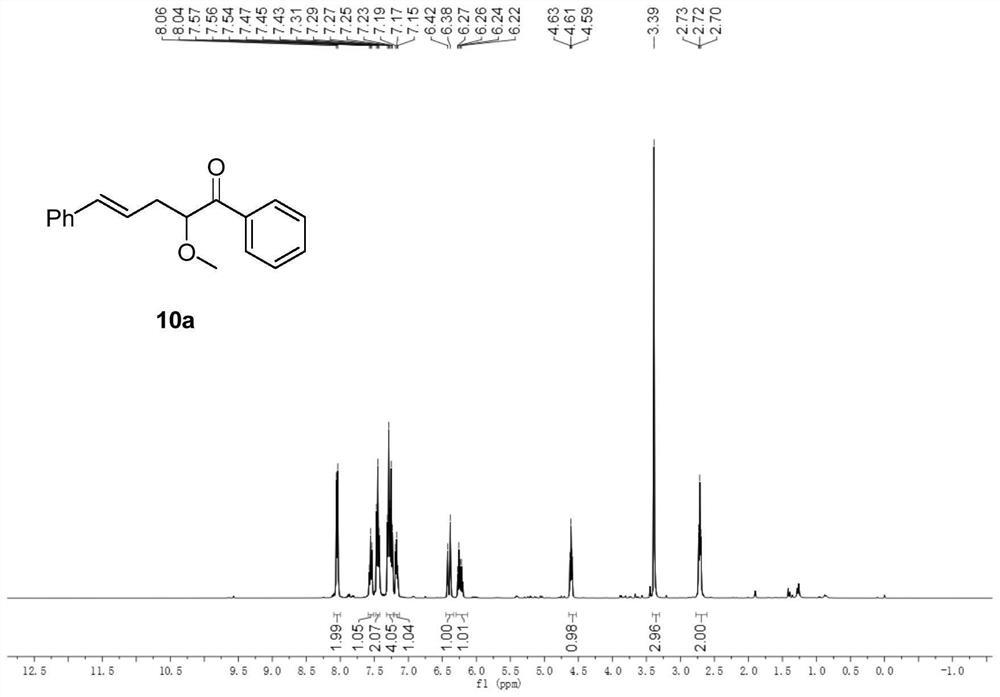
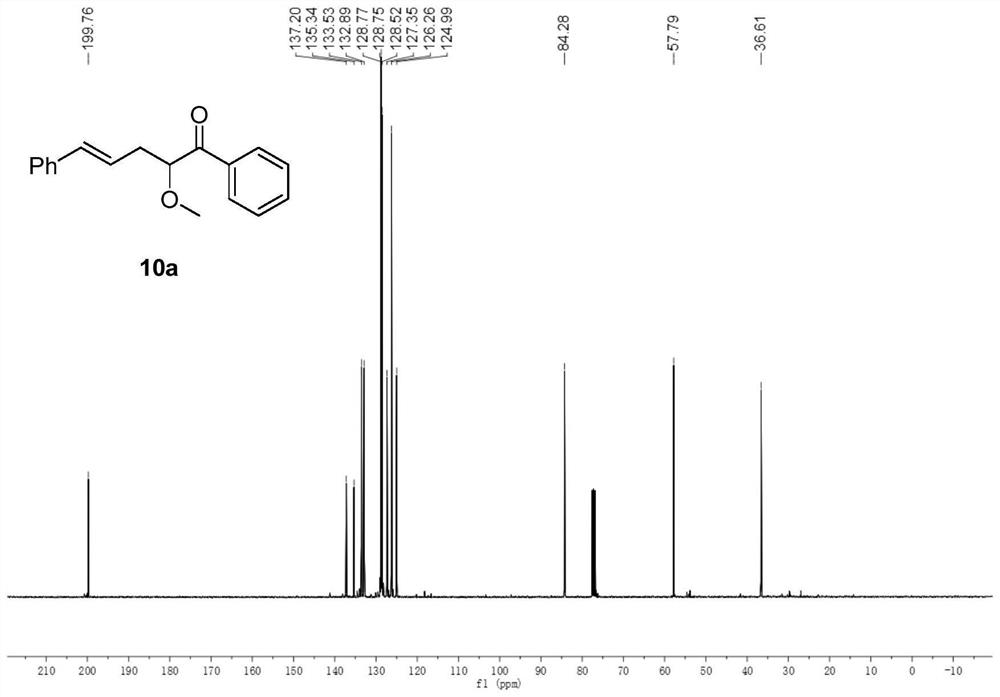
[0053] In a 50ml round bottom flask, add rhodium acetate (0.01mmol), allylpalladium chloride (0.05mmol), 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (0.1 mmol) and under nitrogen protection, cinnamyl methyl carbonate (1 mmol), methanol (1 mmol) were dissolved in 15 ml of dichloromethane and poured into a round bottom flask. Dissolve 2-diazoacetophenone (1.5 mmol) in 5 ml of dichloromethane, pour it into a round bottom flask with a peristaltic pump within 2 hours, and continue the reaction for 0.5 hours after the dropwise addition. After distilling off the solvent under reduced pressure, it was purified by column chromatography (petroleum ether: ethyl acetate = 200:1) to obtain pure product 10a. The yield was 74%. nuclear magnetic resonance 1 HNMR, 13 The C NMR spectrum is shown in FIG. 1 .
[0054] 10a: 1 H NMR (400MHz, CDCl 3 )δ8.05(d, J=7.6Hz, 2H), 7.56(t, J=7.4Hz, 1H), 7.45(t, J=7.6Hz, 2H), 7.32–7.22(m, 4H), 7.17( t,J=7.0Hz,1H),6.40(d,J=15.8Hz,1H),6.25(dd,J=15.2,7....
Embodiment 2
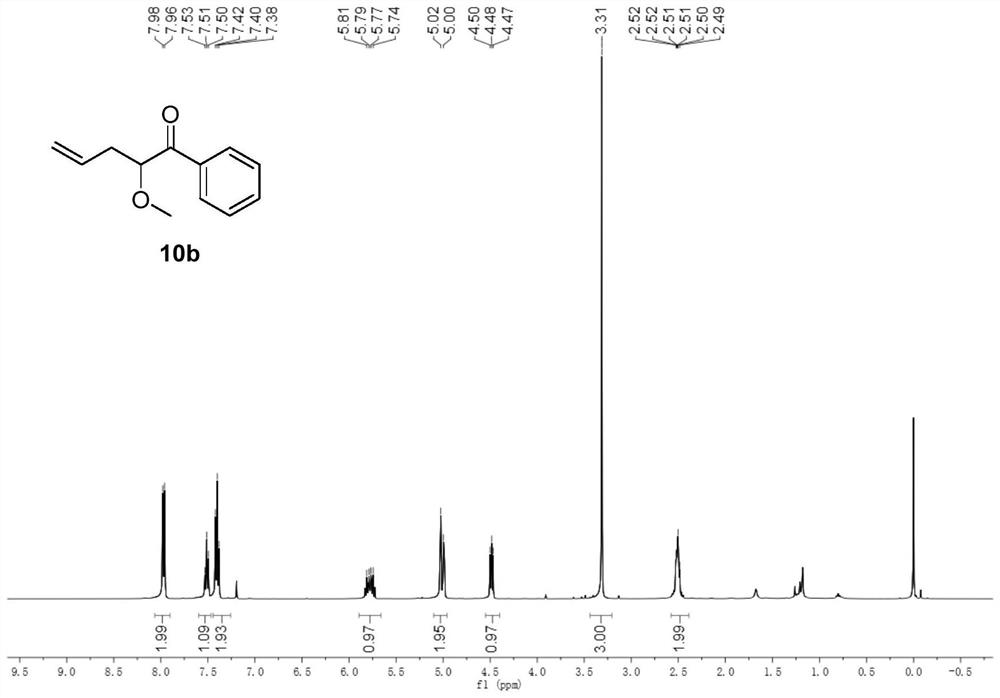
[0059] In a 50ml round bottom flask, add rhodium acetate (0.01mmol), allylpalladium chloride (0.05mmol), 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (0.1 mmol) and under nitrogen protection, allyl methyl carbonate (1 mmol), methanol (1 mmol) were dissolved in 15 ml of dichloromethane and poured into a round bottom flask. Dissolve 2-diazoacetophenone (1.5 mmol) in 5 ml of dichloromethane, pour it into a round bottom flask with a peristaltic pump within 2 hours, and continue the reaction for 0.5 hours after the dropwise addition. After distilling off the solvent under reduced pressure, it was purified by column chromatography (petroleum ether:ethyl acetate=200:1) to obtain pure product 10b. The yield was 62%. nuclear magnetic resonance 1 HNMR, 13 The C NMR spectrum is shown in FIG. 2 .
[0060] 10b: 1 H NMR (400MHz, CDCl 3 )δ7.97(d, J=7.3Hz, 2H), 7.51(t, J=7.4Hz, 1H), 7.40(t, J=7.7Hz, 2H), 5.89–5.66(m, 1H), 5.10– 4.96(m,2H),4.49(dd,J=7.1,5.7Hz,1H),3.31(s,3H),2.58–2.39(m...
Embodiment 3
[0065] In a 50ml round bottom flask, add rhodium acetate (0.01mmol), allylpalladium chloride (0.05mmol), 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (0.1 mmol) and under nitrogen protection, (E)-3-(4-methoxyphenyl)allyl methyl carbonate (1 mmol), methanol (1 mmol) were dissolved in 15 ml of dichloromethane and poured into a round bottom flask. Dissolve 2-diazoacetophenone (1.5 mmol) in 5 ml of dichloromethane, pour it into a round bottom flask with a peristaltic pump within 2 hours, and continue the reaction for 0.5 hours after the dropwise addition. After distilling off the solvent under reduced pressure, it was purified by column chromatography (petroleum ether: ethyl acetate = 200:1) to obtain pure product 10c. The yield was 73%. nuclear magnetic resonance 1 H NMR, 13 The C NMR spectrum is shown in FIG. 3 .
[0066] 10c: 1 H NMR (400MHz, CDCl 3 )δ7.96(d, J=7.7Hz, 2H), 7.48(t, J=7.3Hz, 1H), 7.37(t, J=7.5Hz, 2H), 7.15(d, J=8.4Hz, 2H) ,6.72(d,J=8.3Hz,2H),6.26(d,J=15.8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com