Method for extracting valuable metal from waste lithium ion battery material
A technology for lithium-ion batteries and valuable metals, applied in the field of extracting valuable metals, can solve problems such as secondary pollution, lithium loss, and long process flow, and achieve high-selectivity extraction, inhibition of leaching, and simple process flow Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
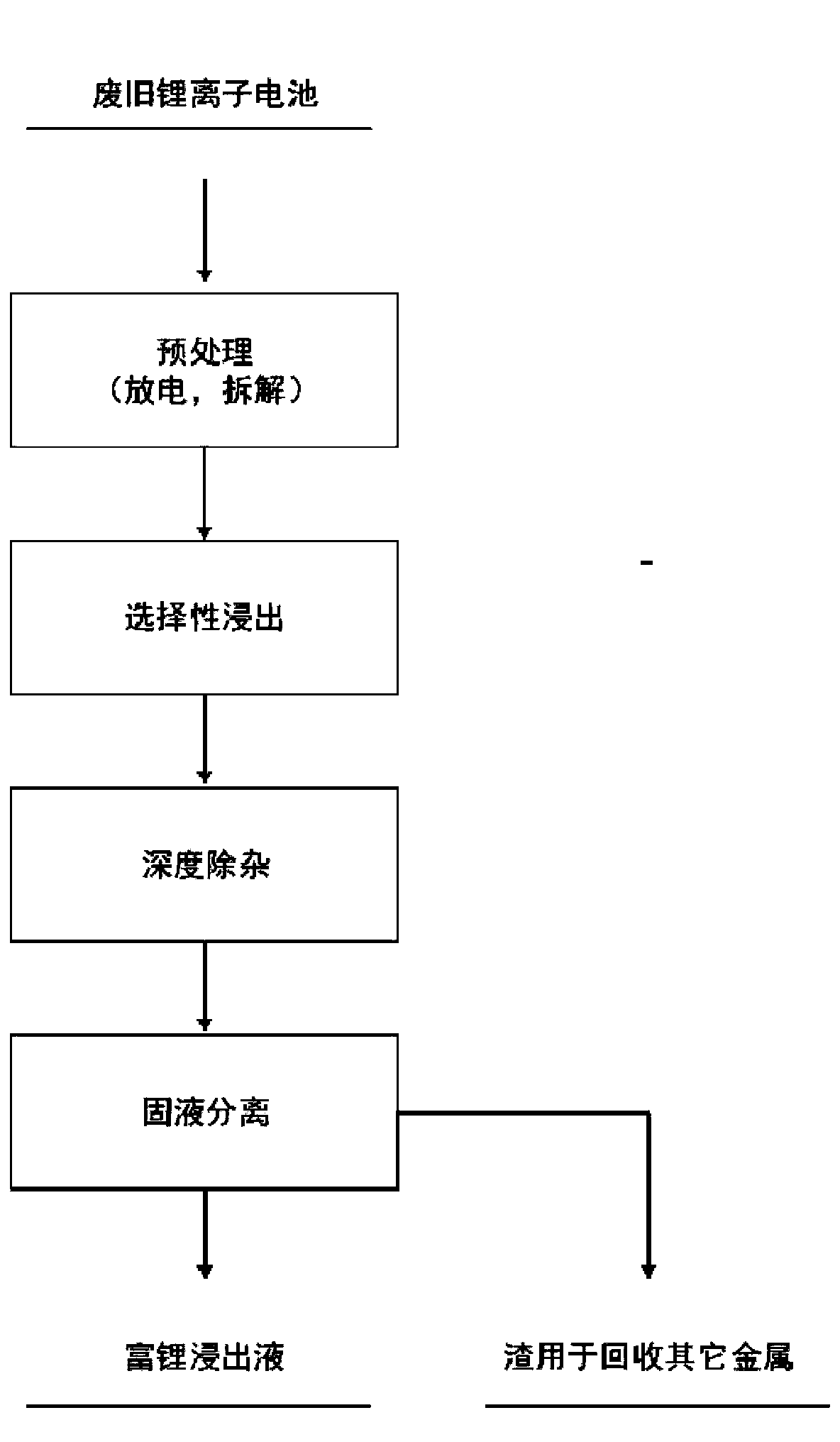
[0059] A method for extracting valuable metals from waste lithium-ion battery materials comprises the steps:
[0060] (1) The 100-mesh battery powder obtained after mechanical crushing and pretreatment (the powder is obtained by mixing and crushing the positive and negative electrodes of waste batteries, the shell, etc., the positive electrode material is nickel-cobalt lithium manganate ternary material, and the negative electrode material Graphite, in addition to aluminum and copper substituted into the positive and negative current collectors, and impurities such as iron introduced during the crushing process) were added to 5wt% sulfuric acid solution, and the solid-to-liquid ratio of the battery powder to the sulfuric acid solution was 200g / L to obtain a mixed material, which is heated and pressurized in an autoclave for 1 hour at a temperature of 180° C., a pressure of 2 Mpa, and a stirring speed of 800 rpm to obtain a leachate;
[0061] (2) Using NaOH to adjust the pH va...
Embodiment 2
[0070] The difference from Example 1 is that the concentration of the sulfuric acid solution in step (1) is 7wt%.
[0071] This example uses the same method as Example 1 to carry out the performance test, and the leaching rate of the obtained lithium element reaches 98%, and the contents of Ni, Co and Mn in the leaching solution are respectively 0.7g / L, 0.3g / L and 0.01g / L , the content of Al, Fe and Cu is respectively 0.001g / L, 0.001g / L and 0.001g / L, and the obtained lithium carbonate product purity is 99.56%, and lithium loss rate is 3%.
Embodiment 3
[0073] The difference from Example 1 is that step (1) is: after the lithium cobalt oxide positive electrode sheet obtained after discharge disassembly is roasted at 500°C for 5h, the obtained waste powder separated from the current collector is added to 5wt % sulfuric acid solution, the solid-to-liquid ratio of the waste powder and sulfuric acid solution is 100g / L, to obtain a mixed material, the mixed material is at a temperature of 200°C, a pressure of 3Mpa, and a stirring speed of 600rpm. Heat and pressurize in an autoclave for 1 hour to obtain a leaching solution.
[0074] This embodiment adopts the method identical with embodiment 1 to carry out performance test, and the obtained lithium element leaching rate reaches 98%, and cobalt content is 0.2g / L in the leaching solution, and the obtained lithium carbonate product purity is 99.56%, and lithium loss rate is 3.2% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com