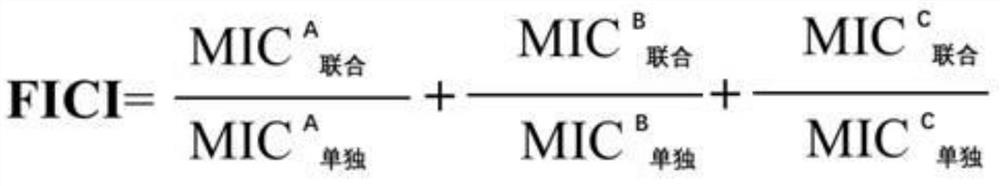Pharmaceutical composition against Pasteurella multocida
A Pasteurella, multi-kill technology, used in antibacterial drugs, pharmaceutical formulations, organic active ingredients, etc., can solve problems such as drug resistance, and achieve the effects of reducing toxic side effects, high bactericidal activity, and good antibacterial effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the combined antibacterial test of pharmaceutical composition to Pasteurella multocida
[0021] 1. Experimental materials and methods
[0022] Reagents and reagents:
[0023] Ciprofloxacin (Ciprofloxacin), Thioridazine (Thioridazine) and Tetracycline (Tetracyclines) were purchased from MCE Company, with purity >98.74%, >99.93%, and >98%, respectively.
[0024] Indicator strain:
[0025] Escherichia coli: Escherichia coli, 25922 (E. coli 25922); standard strain 450, clinically isolated Pa strains: Pa 01, Pa 02, Pa 03, Pa 04, Pa 05, Pa 06, Pa 07, Pa 08, Pa09, Pa 010.
[0026] Medium:
[0027] BHI medium: 10.0g peptone, 12.5g dehydrated calf brain extract powder, 5g dehydrated beef heart extract powder, 5g sodium chloride, 2g glucose, 2.5g disodium hydrogen phosphate in 1000mL distilled water, and adjust the pH to 7.4. The prepared culture medium was autoclaved at 121°C, and after 20 minutes, it was ready for use.
[0028] MH medium: mix 2.0 g of b...
Embodiment 2
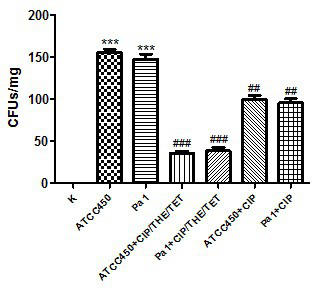
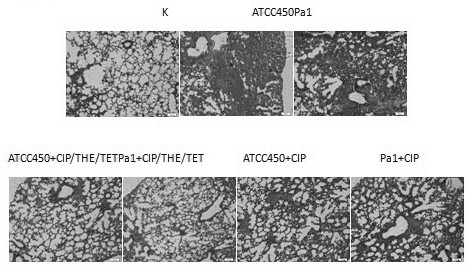
[0050] Embodiment 2: The effect of the drug composition administration treatment on the Pneumonia model of Pasteurella multocida bovine
[0051] 1. Experimental materials and methods
[0052] Reagents and reagents:
[0053] Ciprofloxacin (Ciprofloxacin), Thioridazine (Thioridazine) and Tetracycline (Tetracyclines) were purchased from MCE Company, with purity >98.74%, >99.93%, and >98%, respectively.
[0054] Strains:
[0055] Standard Pa strain 450, clinical isolate Pa strain Pa 1.
[0056] Medium:
[0057] BHI medium: 10.0g peptone, 12.5g dehydrated calf brain extract powder, 5g dehydrated beef heart extract powder, 5g sodium chloride, 2g glucose, 2.5g disodium hydrogen phosphate in 1000mL distilled water, and adjust the pH to 7.4. The prepared culture medium was autoclaved at 121°C, and after 20 minutes, it was ready for use.
[0058] animal
[0059] SPF-grade BALB / C mice, male, weighing 18-22g, were purchased from Changchun Yisi Experimental Animal Co., Ltd. The lab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com