Preparation method of 2,6'-dichlorobenzothiazole
A technology of dichlorobenzothiazole and chlorobenzothiazole, which is applied in the field of preparation of 2,6'-dichlorobenzothiazole, can solve problems that have not been seen, and achieve the effect of simple and green synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
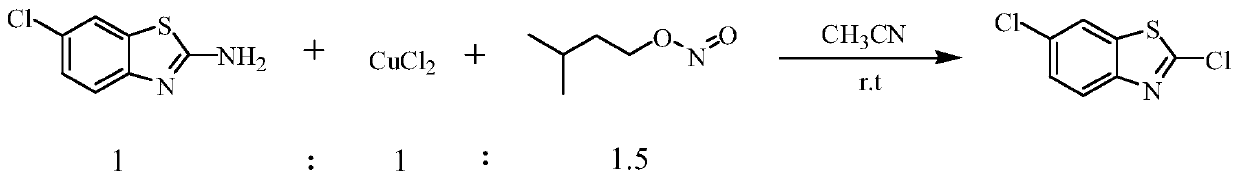
[0015] This embodiment is the preparation method of 2,6'-dichlorobenzothiazole, according to the following reaction formula:
[0016]
[0017] The specific experimental process is as follows:
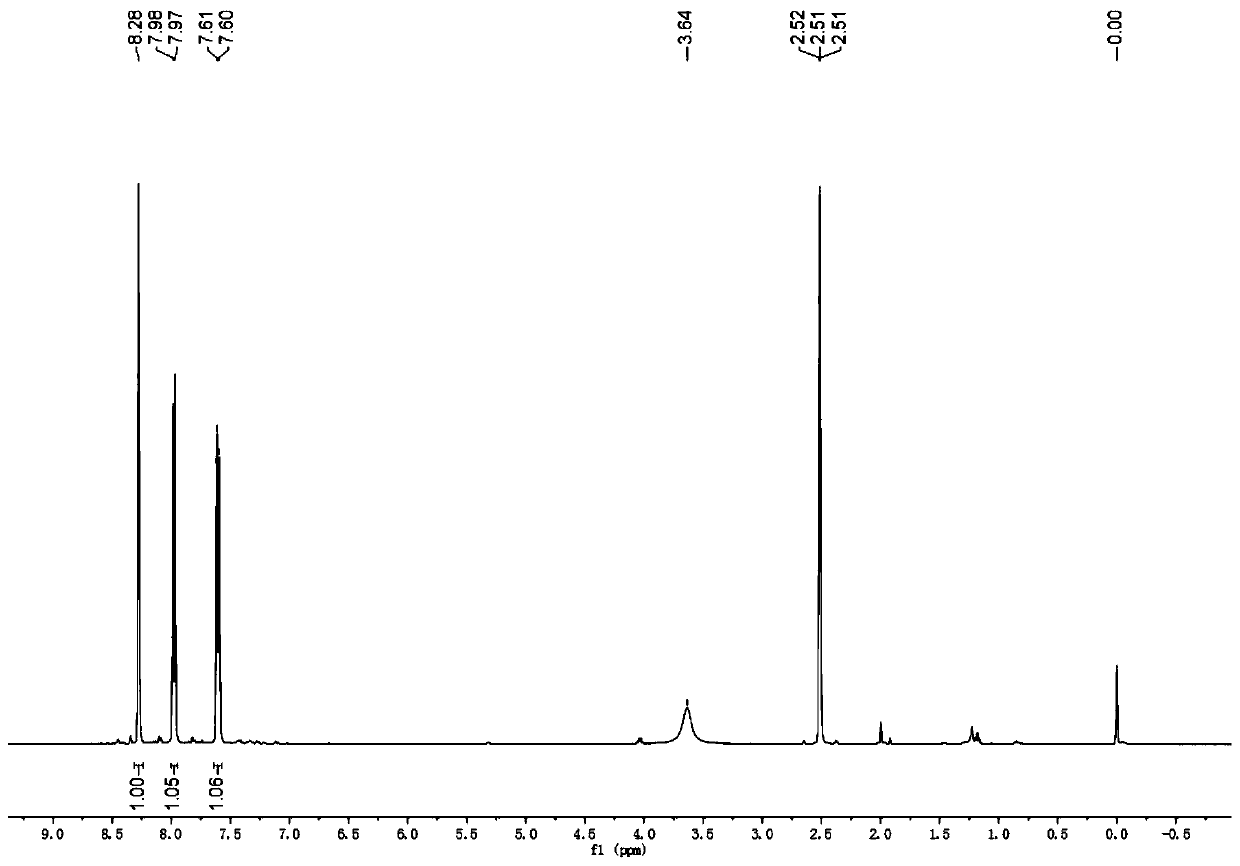
[0018] In a 100ml three-necked flask, 3.69g (0.02mol, 97%, 1eq) of 2-amino-6-chlorobenzothiazole was dissolved in 20ml of acetonitrile, and 2.68g (0.02mol, 1eq) of anhydrous copper chloride was added simultaneously, At room temperature, 3.51 g of isoamyl nitrite (0.03 mol, 98%, 1.5 eq) was added dropwise with magnetic stirring. After the addition, the temperature was raised to reflux, and the reaction was carried out for 2 to 3 hours. The system was a dark green solution with insoluble matter.
[0019] After the reaction was completed, acetonitrile was removed under reduced pressure, and 1.84 g of orange solid 2,6'-dichlorobenzothiazole product was obtained by column chromatography, GC: 97%, and the separation yield was 45%.
Embodiment 2
[0021] This embodiment is the preparation method of 2,6'-dichlorobenzothiazole, according to the following reaction formula:
[0022]
[0023] The specific experimental process is as follows:
[0024] In a 100ml three-necked flask, 3.69g (0.02mol, 97%, 1eq) of 2-amino-6-chlorobenzothiazole was dissolved in 20ml of acetonitrile, and 2.68g (0.03mol, 1eq) of anhydrous copper chloride was added simultaneously, At room temperature, 54 g of isoamyl nitrite (0.03 mol, 98%, 1.5 eq) was added dropwise under magnetic stirring. After the addition, the temperature was raised to reflux, and the reaction was carried out for 2 to 3 hours. The system was a dark green solution with insoluble matter.
[0025] After the reaction was completed, the acetonitrile was removed under reduced pressure, and 20 mL of ethyl acetate was added to the residue obtained from the still, washed with saturated sodium chloride, separated into layers, the organic layer was dried with anhydrous magnesium sulfate,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com