Preparation method and application of cytarabine-modified methotrexate-loaded liposome
A technology of carrying methotrexate lipids and carrying methotrexate, which is applied in the field of biomedicine and can solve the problems of high disease recurrence rate, low specificity of methotrexate, and serious side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
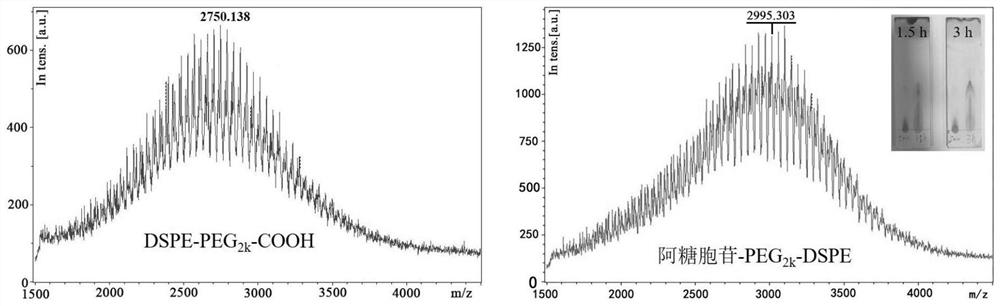
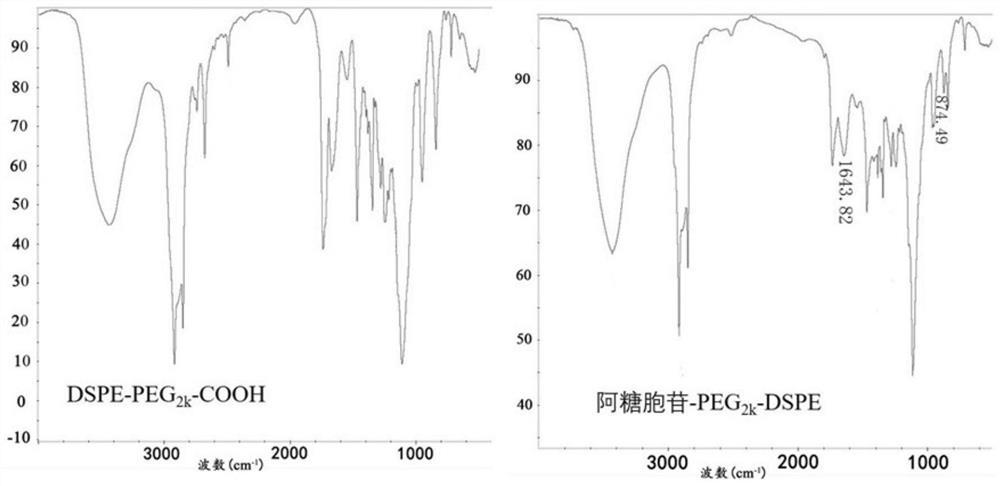
[0029] 1. Cytarabine-PEG 2k -DSPE synthesis:
[0030] (1) Precisely weigh 140.00mg DSPE-PEG 2k -COOH (MW: 2800), dissolve in 10ml of dimethylformamide and shake well. Then accurately weighed 14.60mg cytarabine (MW: 243.22), 22.80mg 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethylurea hexafluorophosphate ( HATU), triethylamine, and 1-hydroxybenzotriazole (HOBt) were sequentially added to the above dimethylformamide solution system, and stirred in an ice bath for 3 hours, where DSPE-PEG 2k The molar ratio of COOH, cytarabine, HATU, HOBt and triethylamine is 1:1.2:1.2:1.2:2.
[0031] (2) After (1) the reaction is completed, the reaction system is moved to a dialysis bag with a molecular weight cut-off of 3500 Da, and dialyzed in 1000 ml of deionized water for 72 hours, changing the deionized water every 6 hours.
[0032] (3) After the dialysis, the liquid in the dialysis bag is cooled by program and freeze-dried for 48 hours to obtain Cytarabine-PEG 2k -DSPE.
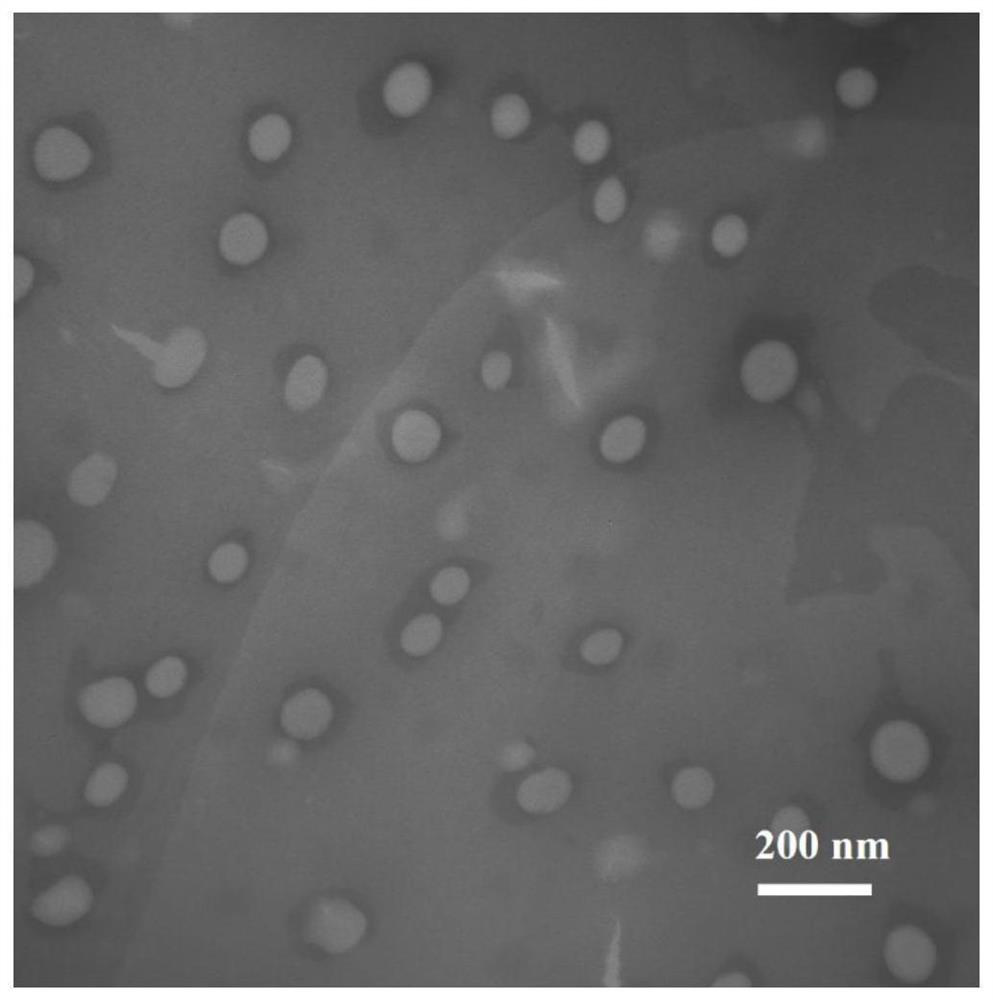
[0033] 2. Preparation of c...
Embodiment 2
[0043] 1. Cytarabine-PEG 2k -DSPE synthesis:
[0044] (1) Precisely weigh 140.00mg DSPE-PEG 2k -COOH (MW: 2800), dissolve in 10ml of dimethylformamide and shake well. Then accurately weighed 14.60mg cytarabine (MW: 243.22), 22.80mg 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethylurea hexafluorophosphate ( HATU), triethylamine, and 1-hydroxybenzotriazole (HOBt) were sequentially added to the above dimethylformamide solution system, and stirred in an ice bath for 3 hours, where DSPE-PEG 2k The molar ratio of COOH, cytarabine, HATU, HOBt and triethylamine is 1:2:1.2:1.2:2.
[0045] (2) After (1) the reaction is completed, the reaction system is moved to a dialysis bag with a molecular weight cut-off of 3500 Da, and dialyzed in 1000 ml of deionized water for 72 hours, changing the deionized water every 6 hours.
[0046] (3) After the dialysis, the liquid in the dialysis bag is cooled by program and freeze-dried for 48 hours to obtain Cytarabine-PEG 2k -DSPE.
[0047] 2. Preparation of cyt...
Embodiment 3
[0055] 1. Cytarabine-PEG 2k -DSPE synthesis:
[0056] (1) Precisely weigh 140.00mg DSPE-PEG 2k -COOH (MW: 2800), dissolve in 10ml of dimethylformamide and shake well. Then accurately weigh 14.60mg cytarabine (MW: 243.22), 22.80mg 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethylurea hexafluorophosphate ( HATU), triethylamine, and 1-hydroxybenzotriazole (HOBt) were added to the above dimethylformamide solution system in sequence, and stirred in an ice bath for 5 hours, where DSPE-PEG 2k The molar ratio of COOH, cytarabine, HATU, HOBt and triethylamine is 1:1.2:1.2:1.2:2.
[0057] (2) After (1) the reaction is completed, the reaction system is moved to a dialysis bag with a molecular weight cut-off of 3500 Da, and dialyzed in 1000 ml of deionized water for 72 hours, changing the deionized water every 6 hours.
[0058] (3) After the dialysis, the liquid in the dialysis bag is cooled by program and freeze-dried for 48 hours to obtain Cytarabine-PEG 2k -DSPE.
[0059] 2. Preparation of cyta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com