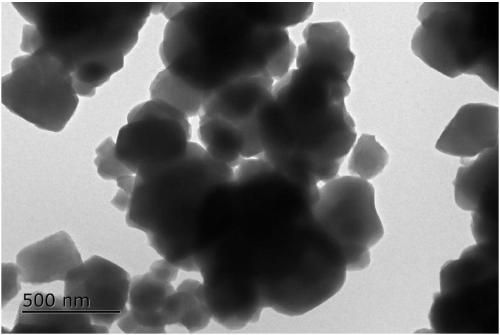
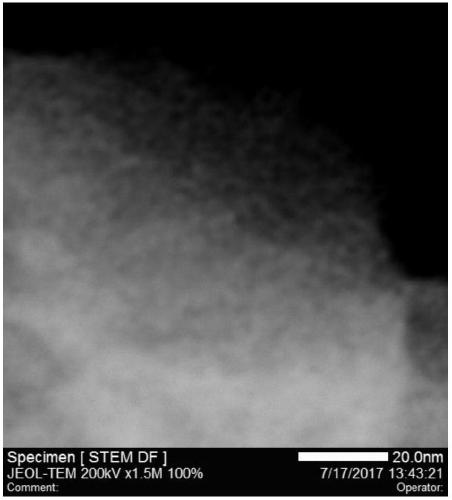
Preparation method of loaded nano-metal catalyst based on UIO-66
A UIO-66, metal catalyst technology, applied in the direction of metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the long and cumbersome catalyst synthesis steps, catalyst adverse effects, Stabilizer is difficult to remove and other problems, to achieve the effect of high industrial use value, simple preparation method, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of Rh@CeO2@C Catalyst
[0025] Weigh 9.36g of cerium ammonium nitrate and dissolve it in 22ml of water, dissolve 2.82g of terephthalic acid in 91ml of N,N-dimethylformamide, after ultrasonic dissolution, mix the two solutions evenly, and keep stirring at 100°C , add 1.00g of rhodium chloride solution (10mgRh / mL), continue to stir for 20min, filter, wash with N,N-dimethylformamide three times, wash three times with water, wash three times with ethanol, and bake at 60°C for 12h. Rh@UIO-66(Ce) material; after grinding the Rh@UIO-66(Ce) material, the Rh@UIO-66(Ce) sample was transferred to a tube furnace, and the temperature was raised to a temperature of 5°C / min. The pyrolysis temperature was 600 °C, and it was pyrolyzed for 5 h in a nitrogen atmosphere. After natural cooling, the Rh@CeO2@C precursor was obtained, and the Rh@CeO2@C catalyst was obtained after reduction.
Embodiment 2
[0027] Preparation of Pt@CeO2@C Catalyst
[0028] Dissolve 9.36g of ceric ammonium nitrate in 22ml of water and 2.82g of terephthalic acid in 91ml of N,N-dimethylformamide. After ultrasonically dissolving, mix the two solutions evenly and keep stirring at 100°C. Add 0.50g chloroplatinic acid solution (20mgPt / mL), continue stirring for 20min, filter, wash with N,N-dimethylformamide three times, wash three times with water, wash three times with ethanol, and bake at 60°C for 12h to obtain Pt @UIO-66(Ce) material; after grinding the Pt@UIO-66(Ce) material, the Pt@UIO-66(Ce) sample was transferred to a tube furnace, and the temperature was raised to the heat at a heating rate of 5°C / min. The decomposition temperature was 800 °C, and it was pyrolyzed for 5 h in a nitrogen atmosphere. After natural cooling, the Pt@CeO2@C precursor was obtained, and the Pt@CeO2@C catalyst was obtained after reduction.
Embodiment 3
[0030] Preparation of Ir@ZrO2@C Catalyst
[0031] Dissolve 3.98g of zirconium tetrachloride in 22ml of water and 2.82g of terephthalic acid in 91ml of N,N-dimethylformamide. After ultrasonically dissolving, mix the two solutions evenly and keep stirring at 100°C. , add 0.50g chloroiridic acid solution (20mgIr / mL), continue to stir for 20min, filter, wash with N,N-dimethylformamide three times, wash three times with water, wash three times with ethanol, and bake at 60°C for 12h to get Ir@UIO-66(Zr) material; after grinding the Ir@UIO-66(Zr) material, the Ir@UIO-66(Zr) sample was transferred to a tube furnace, and the temperature was raised to a temperature of 5°C / min. The pyrolysis temperature was 800 °C, and it was pyrolyzed for 5 h in a nitrogen atmosphere. After natural cooling, the Ir@ZrO2@C precursor was obtained, and the Ir@ZrO2@C catalyst was obtained after reduction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com