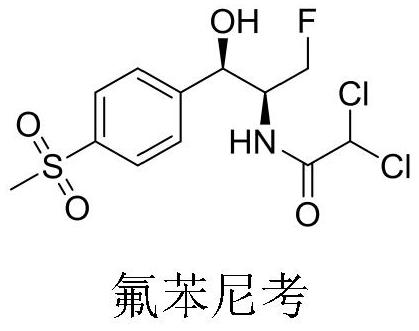
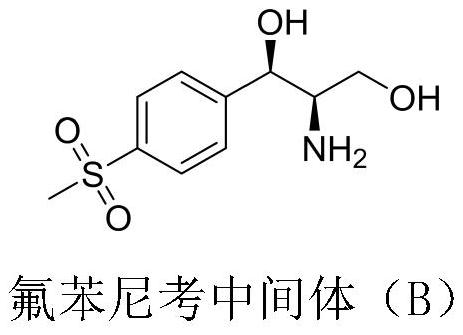
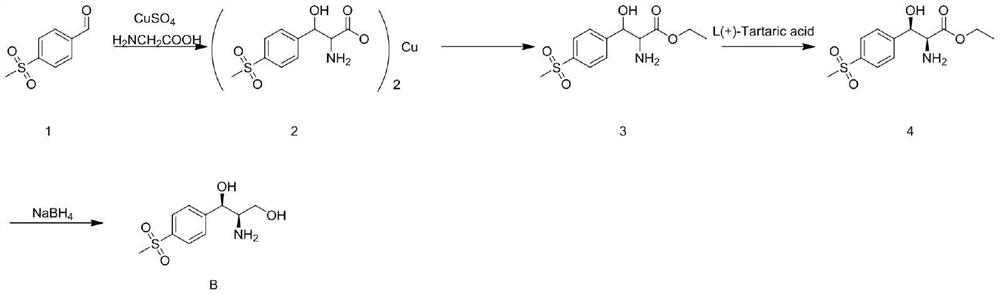
A kind of preparation method of Florfenicol intermediate
A florfenicol and intermediate technology, which is applied in the field of chemical synthesis, can solve the problems of long synthesis steps, low overall yield, complicated operation and the like of florfenicol intermediate B, and achieves high atom utilization rate and short process route. , the effect of simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] The invention discloses a preparation method of Florfenicol intermediate (1R, 2R)-2-amino-1-(4-methylsulfonyl)phenyl)propane-1,3-diol, which uses p-methylsulfone Phenylbenzaldehyde is used as a starting material, and it is prepared through asymmetric addition reaction and hydrogenation reaction. The preparation route is as follows:
[0029]
[0030] The present invention adopts p-thiamphenicyl benzaldehyde and nitroethanol in Cu(OTf) 2 Synthesis of (1R, 2R)-1-(4-(methylsulfonyl)phenyl)-2-nitropropane-1,3-diol (compound A) under the catalysis of / L complex, and then add Reaction after hydrogen affords Florfenicol intermediate (1R, 2R)-2-amino-1-(4-methylsulfonyl)phenyl)propane-1,3-diol (compound B), the Cu( OTf) 2 / L catalyst is copper salt complex, copper salt complex Cu(OTf) 2 / L by Cu(OTf) 2 Prepared by refluxing the toluene solution and ligand L in alcohol, Cu(OTf) 2 The molar ratio with ligand L is 2:1, and the structure of ligand L is as follows:
[0031] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com