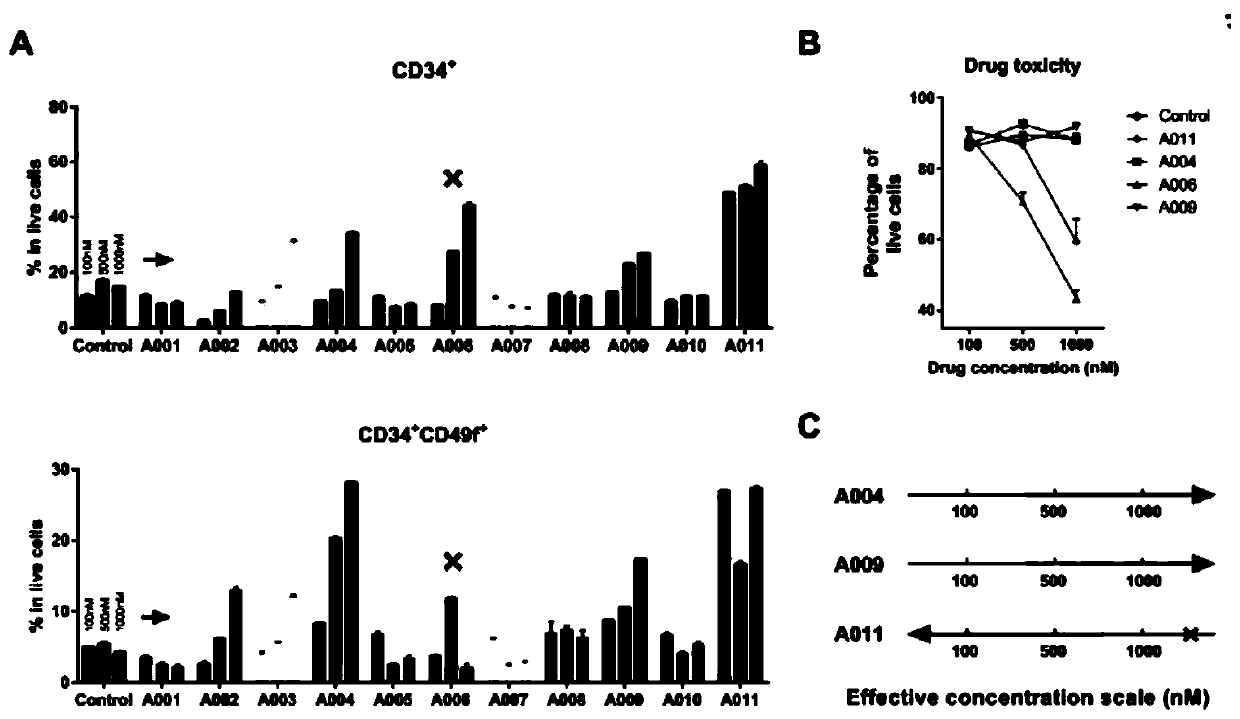
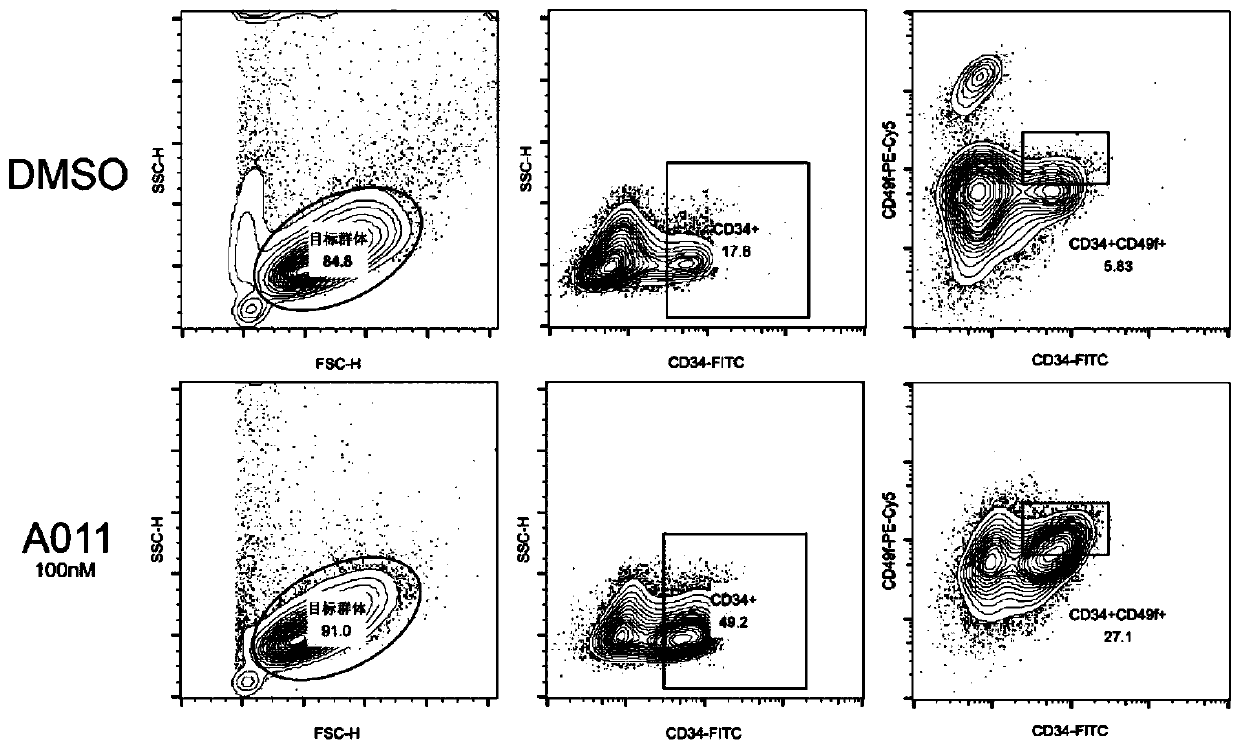
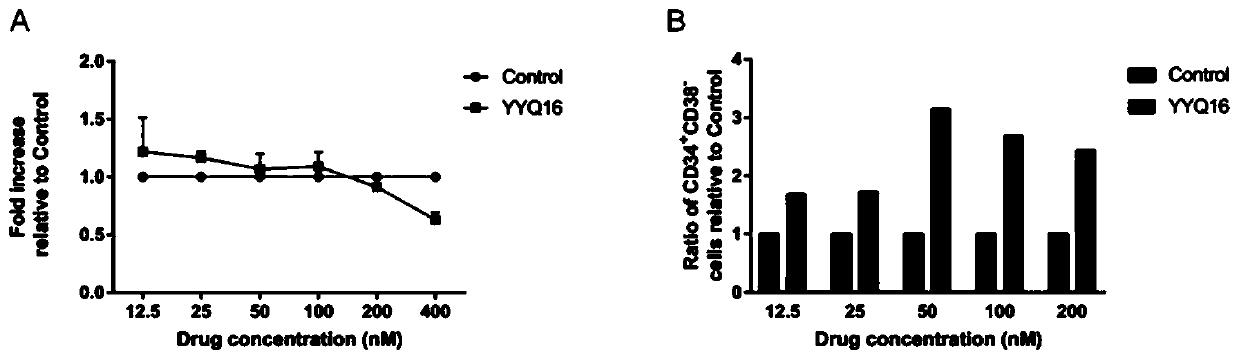
Pyrimidinoindole compound as well as preparation method and application thereof
A technology for compounds, pyrimidines, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1. Intermediate 5-(3-nitrophenyl) tetrazole (B1) synthesis
[0054] Take 30.0g (202.69mmol) of m-nitrobenzonitrile, 14.5g (222.96mmol) of sodium azide, and 45.7g (202.69mol) of zinc bromide in a 1000ml flask, add 500ml of water, reflux at 100°C for 24h, then cool down To room temperature, pour the reaction solution into 500ml of water, add 100ml of 3N hydrochloric acid, extract with ethyl acetate (3×200ml), collect the organic phase, spin dry the ethyl acetate under reduced pressure to obtain a white solid, add 800ml of 0.25mol / L NaOH, stirred, the solid dissolved first and then re-precipitated white flocculent zinc hydroxide, suction filtered, the filtrate was adjusted to pH=5 with 3.0mol / L hydrochloric acid, a large amount of white solid was precipitated, and the dry white B1 product was 34.8g, the yield was 90 %. 1 H NMR (DMSO-d 6 , ppm) δ8.85-8.86 (t, 1H), 8.48-8.51 (dt, 1H), 8.43-8.46 (dq, 1H), 7.91-7.95 (t, 1H).
Embodiment 2
[0055] Embodiment 2. the synthesis of intermediate 2-methyl-5-(3-nitrophenyl) tetrazole (B2)
[0056] Take 33.0g (172.64mmol) of intermediate B1 and dissolve it in 150ml DMF, add 28.63g (207.17mmol) of anhydrous potassium carbonate, 29.41g (207,17mmol) of methyl iodide, react at 90°C for 1h, cool to room temperature, and the reaction solution has Dark yellow, the reaction solution was slowly dropped into 1L of water, a large amount of white solid was precipitated, filtered by suction, and dried to obtain a white solid. Recrystallization with ethyl acetate and petroleum ether gave 26 g of product B2, with a yield of 72.2%. 1 H NMR (DMSO-d 6 , ppm) δ8.66-8.67 (t, 1H), 8.40-8.42 (dt, 1H), 8.33-8.35 (dt, 1H), 7.81-7.85 (t, 1H), 4.46 (s, 3H).
Embodiment 3
[0057] Example 3. Synthesis of intermediate N-hydroxyl-N-acetyl-4-(2-methyltetrazol-5-yl)aniline (B4)
[0058] Take 30g (146.22mmol) of intermediate B2, dissolve it in 350ml tetrahydrofuran at room temperature, add 10.0g NI powder, cool down to 0°C in an ice bath, slowly drop in 7.3g (143,49mmol) of hydrazine hydrate in batches, and stir at 0°C After 30 minutes, slowly warm up to room temperature and continue to react for 24 hours. The solution turns light yellow. After TLC monitors that the raw materials disappear, cool down to 0°C, add 4 equivalents of sodium bicarbonate 54g, gas is generated, stir for 30mim, and drop in acetyl chloride 13.77g at 0°C. g (175.46mmol), slowly warmed up to room temperature for 2h, suction filtered, and the filter cake was washed with THF (3×50ml), the filtrate was collected, and THF was spin-dried under reduced pressure to obtain a yellow solid. The solid was recrystallized from ethyl acetate to obtain 28 g of product B4 with a yield of 84%. 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com