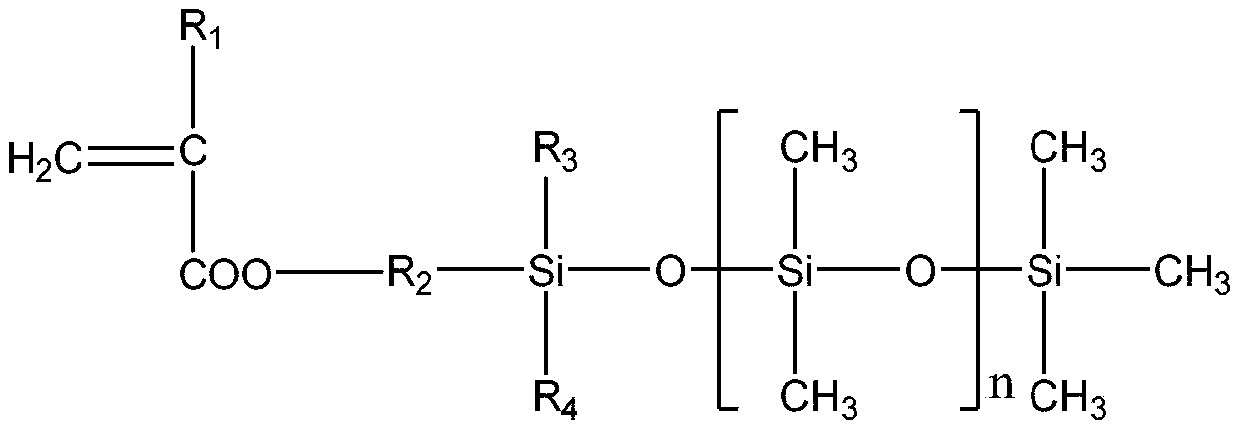
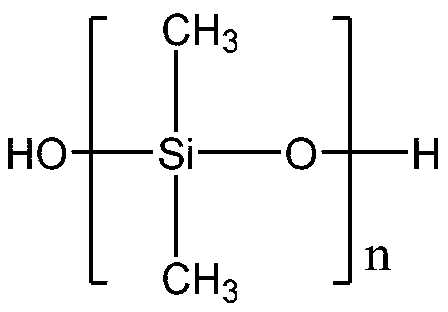
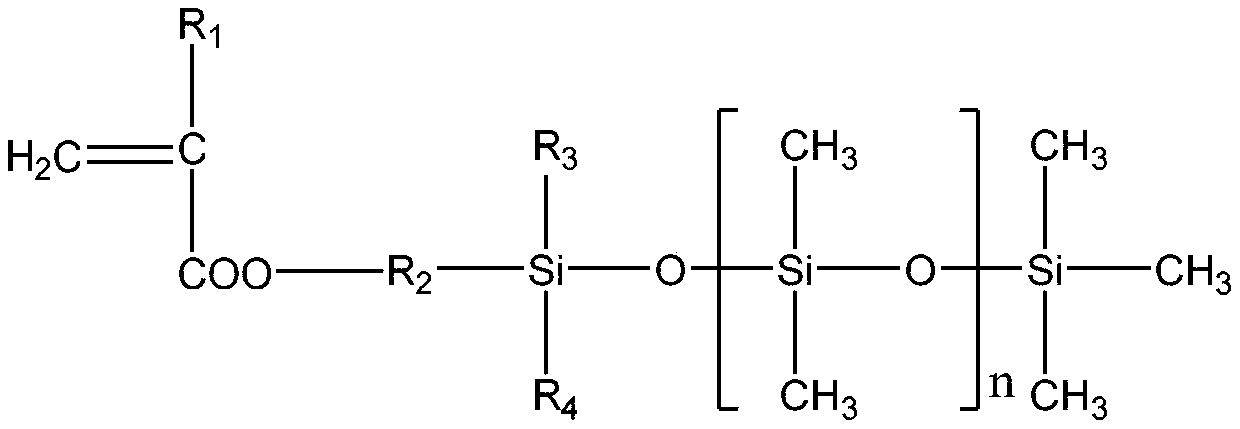
Single-end-capping reactive organosilicon
A reactive silicone, single-end capping technology, applied in the direction of film/sheet adhesive, film/sheet release liner, adhesive, etc., can solve the problems of increased raw material cost and difficult control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] In the preparation method of the single-end capped reactive organosilicon of the present invention, the following compounds are commonly used as reaction catalysts:
[0024] Commonly used organotin compounds include: dibutyltin dilaurate, dibutyltin diacetate, dibutyltin diethylhexanoate, dibutyltin dioctoate, dibutyltin dimethylmaleate, dibutyltin maleate, dioctyltin diacetate , Dioctyltin distearate, Dioctyltin dilaurate, Dibutyltin dimethyl, Dibutyltin diphenoxy, Dibutyltin dibutylketoxime, Dibutyltin diacetylacetonate, Dibutyltin diacetoacetate, Tetravalent tin compounds such as dibutyltin bistriethoxysilicate, dioctyltin bistriethoxysilicate and reaction products of diketoxime tin oxide and silicate compounds; stannous octoate, tin naphthenate, Divalent tin compounds such as tin stearate; butyl tin or monooctyl tin compounds such as monobutyl tin trioctanoate, butbutyl tin triisopropoxide, or one or a mixture of the above-mentioned organotins.
[0025] Commonly us...
Embodiment 1
[0030] [Example 1] 100 parts by weight of hydroxyl silicone oil with a viscosity of 200mPa.s, 5 parts by weight of methacryloxypropyltrimethoxysilane, and 0.05 parts by weight of dibutyltin dilaurate are added to the 2 In the three-necked flask of the protected cooling reflux device, heat the bottom with an oil bath until the temperature in the flask is 80°C, and test the viscosity of the reaction solution every 10 minutes. Once the viscosity reaches 300mPas, add 3 parts by weight of hexamethyldisilazane immediately, and continue Heat the reaction for 1 hour until the residual hydroxyl group completely disappears (the FTIR confirms that there is no hydroxyl peak), and after the reaction is completed, the small molecule alcohol reactant is distilled off under reduced pressure to obtain a single-terminal methacryloyloxy polydicarboxylate with a viscosity of 295 mPas. Methylsiloxane.
Embodiment 2
[0031] [Example 2] 100 parts by weight of hydroxyl silicone oil with a viscosity of 500mPa.s, 4 parts by weight of acryloxypropyltrimethoxysilane, and 0.1 part by weight of bismuth octoate are added to the 2 In the three-necked flask of the protected cooling reflux device, heat it with an oil bath until the temperature in the flask is 80°C. Test the viscosity of the reaction solution every 10 minutes. Once the viscosity reaches 700mPas, add 2.5 parts by weight of hexamethyldisilazane immediately, and continue Heat the reaction for 1 hour until the residual hydroxyl group completely disappears (the FTIR confirms that there is no hydroxyl peak). After the reaction is completed, the small molecule alcohol reactant is removed by distillation under reduced pressure, and finally a polydimethylmethylene with a single-end acryloyloxy group with a viscosity of 691 mPas is obtained. base siloxane.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com