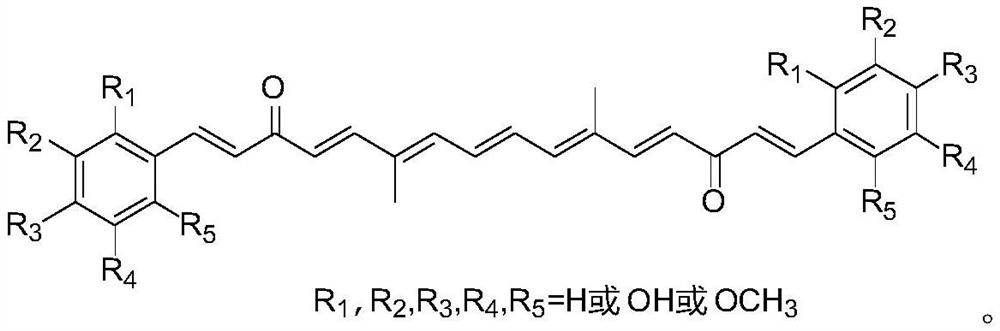
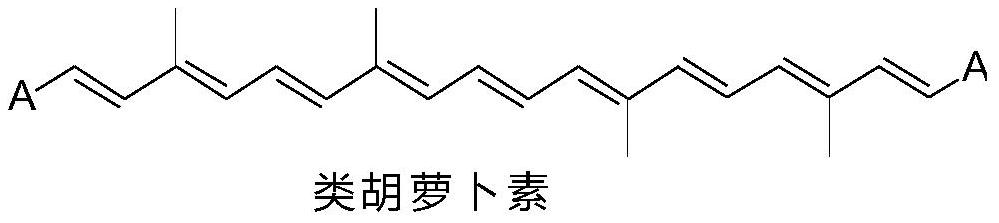
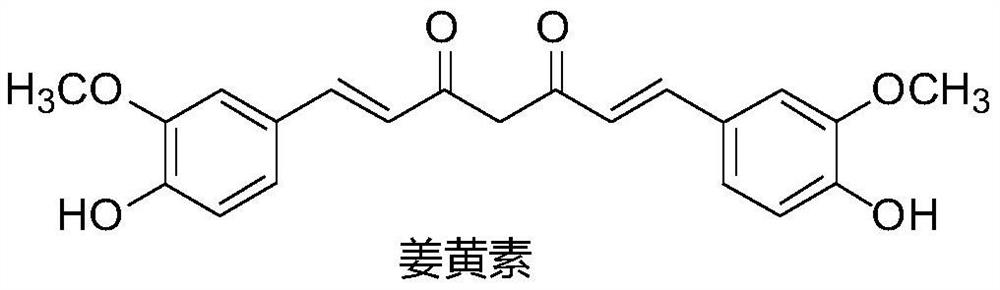
A Class of Polyenediones Antitumor Compounds
A technology for polyene dione and antitumor drug, applied in the field of polyene dione compounds, can solve the problems of poor anticancer activity, fast metabolism of curcumin, low absorption degree and the like, and achieves mild preparation conditions, high yield, Strong inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The in vitro free radical scavenging test of embodiment 1 target compound
[0036] Accurately weigh 19.7mg of DPPH, dissolve it with 450mL of absolute ethanol ultrasonically and dilute it to a 500mL volumetric flask to prepare a 0.1mM DPPH solution, and store it in the refrigerator in the dark for future use.
[0037] The configuration and experimental operation of the sample to be tested are as follows: Weigh 10.0 mg of the sample to be tested, add absolute ethanol to make a 10 mg / L mother solution, and the corresponding molar concentration is about 16-20 μmol / L. Use a pipette gun to accurately measure 100 μL of the sample to be tested and 200 μL of absolute ethanol in the microplate plate, set the background on the microplate reader for zero adjustment, to eliminate the influence of the sample on the test results; draw 100 μL of distilled water and 200 μL of 0.1mM DPPH solution was used as a blank control group; 100 μL of the solution to be tested and 200 μL of the DP...
Embodiment 2
[0040] Antitumor activity test of embodiment 2 target compound
[0041] Culture medium preparation: 89% culture medium + 10% fetal bovine serum + 1% double antibody.
[0042] PBS preparation: accurately weigh 3.61g Na 2 HPO 4 12H 2 O, 0.2g KCl, 0.8g NaCl and 0.2g KH 2 PO 4 , add 800mL double distilled water to dissolve completely, then dilute to 1000mL with double distilled water. Pack in glass bottles for autoclaving and store in a 4°C refrigerator for later use.
[0043] Preparation of MTT mother liquor: Accurately weigh 250mg MTT, dissolve in 50mL PBS in the dark, shake gently in a water bath at 60°C to dissolve completely, filter through a 0.22μm microporous filter membrane, and dispense at -20°C Store in dark place.
[0044] Drug preparation: Accurately weigh 9 target compounds according to the concentration in the MTT experiment in Table 3, add 200uL DMSO to dissolve completely, and store in a refrigerator at 4°C in the dark.
[0045] Table 3 Dosing concentration...
Embodiment 3
[0053] The preparation of embodiment 3 target object
[0054] At 0° C., in a mixed system of 1 mL of acetone and 2 mL of NaOH (15%) aqueous solution, slowly dropwise add an ethanol solution (5 mL) of substituted benzaldehyde (2 mmol), and react at room temperature after the addition is complete. After the reaction of the raw materials was detected by TLC, the solvent was removed by rotary evaporation, and dichloromethane was added to completely dissolve the solid crude product in the bottle, and then the pH was adjusted to neutral with saturated ammonium chloride solution, washed with saturated brine, and dried with anhydrous magnesium sulfate. After separation by column chromatography (petroleum ether / ethyl acetate=10 / 1, V / V), the substituted methyl styrene acetone was obtained in the form of white powder or white oil, with a yield of 75-89%. Then, under the protection of nitrogen, dry and ground KOH (0.44g, 0.08mmol) was added to the obtained mixed solution of substituted me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com