LA-GFLG-DOX conjugate, preparation method and use thereof
A technology of LA-GFLG-DOX and conjugates, applied in the field of LA-GFLG-DOX conjugates and their preparation, to achieve the effect of reducing damage and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
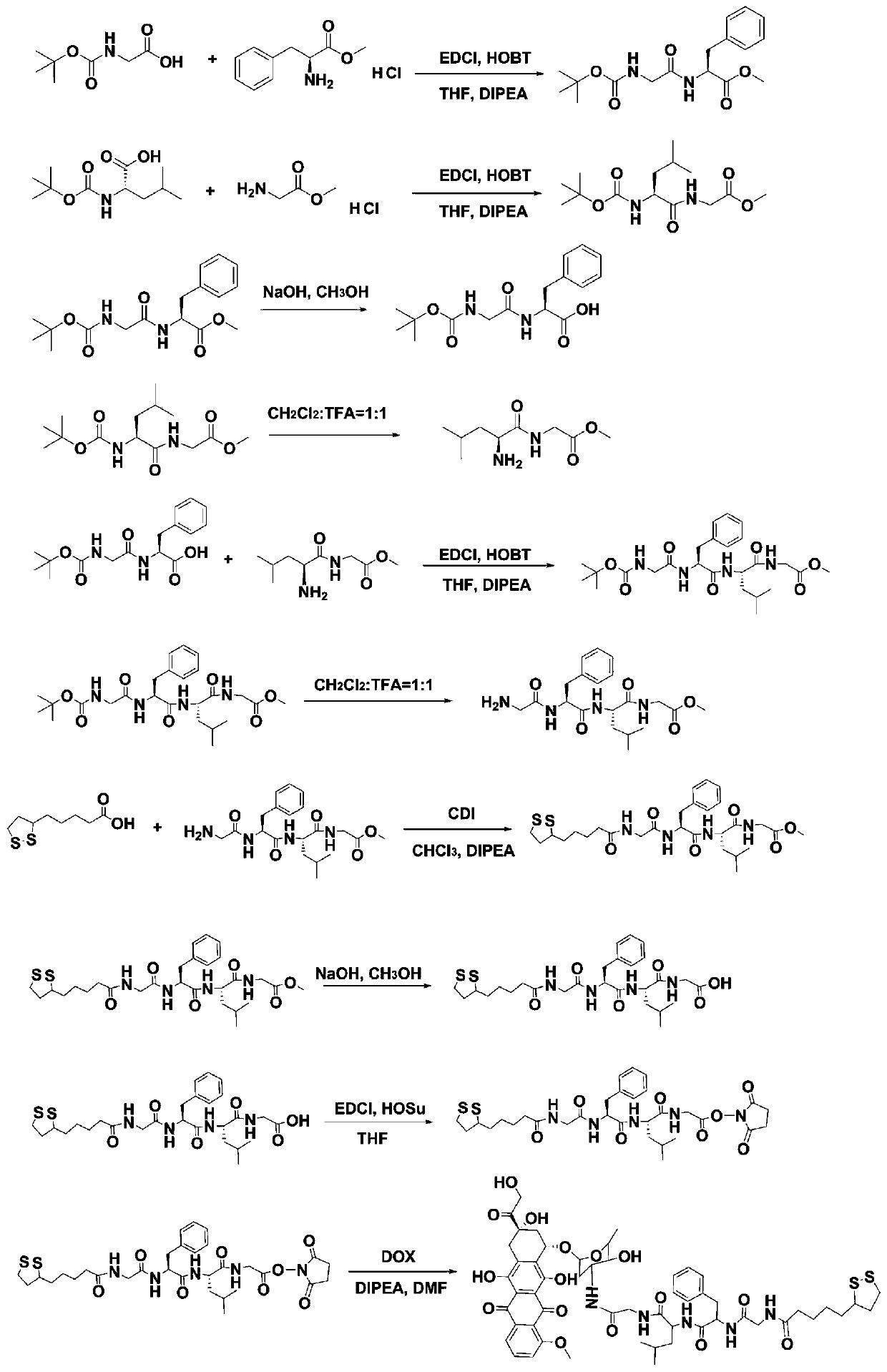
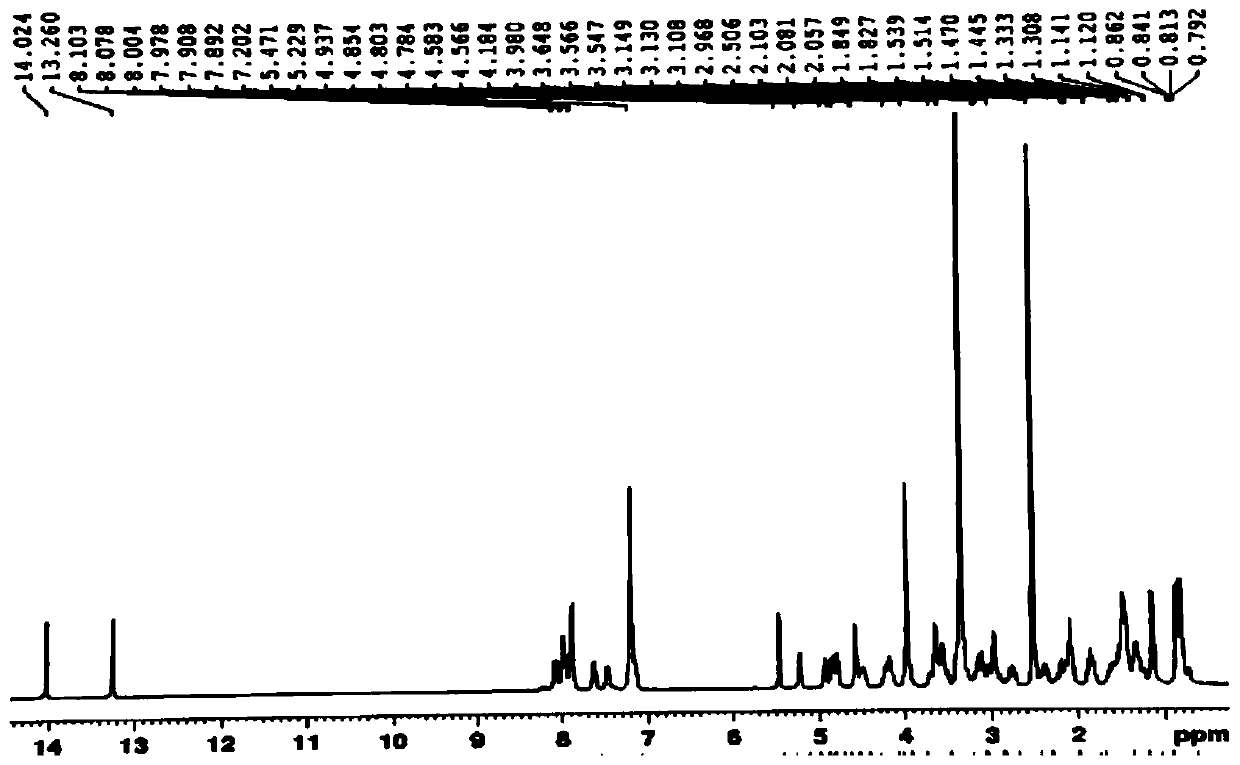
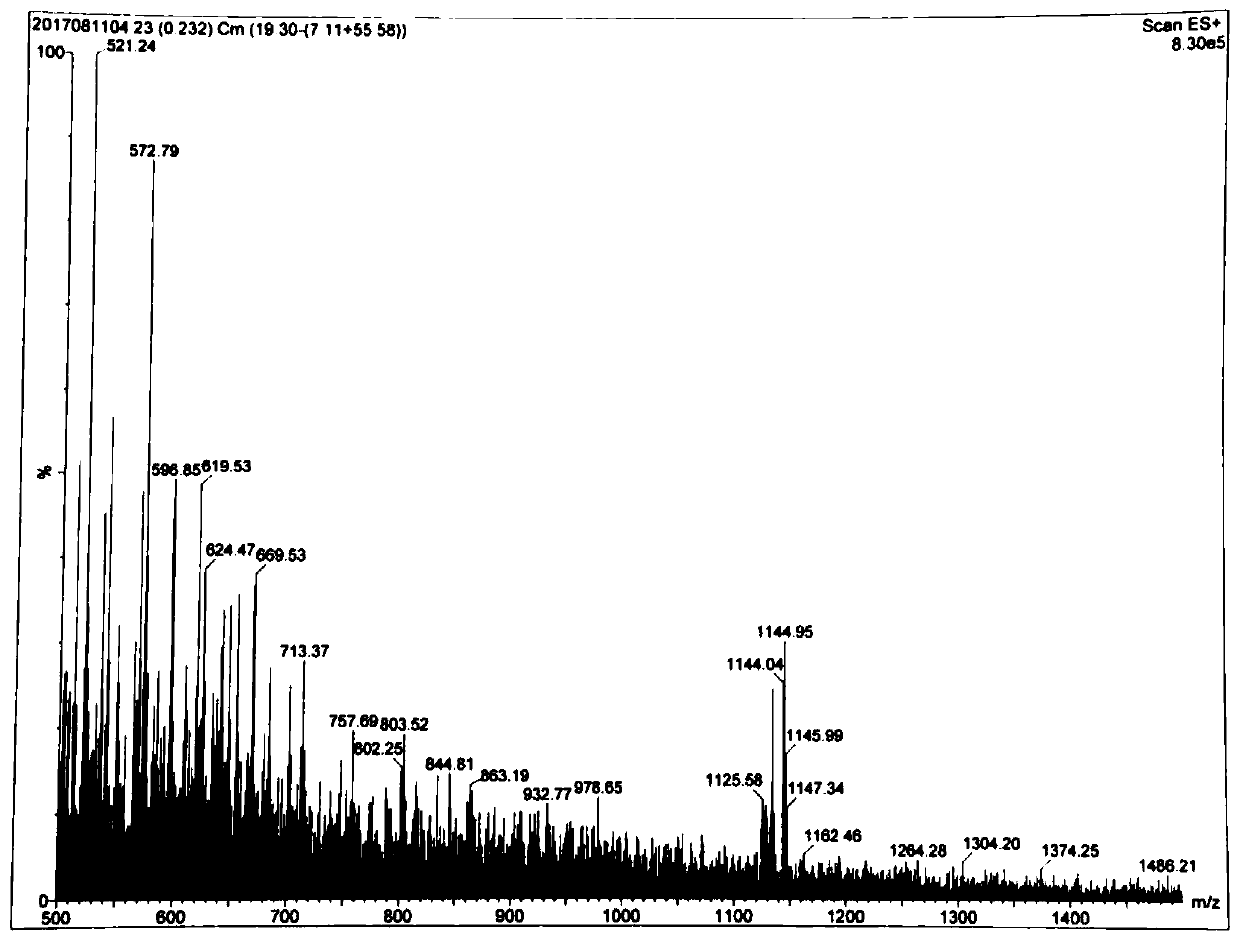
[0035] 1. Preparation of LA-GFLG-DOX conjugates
[0036] Synthetic route such as figure 1 Shown:
[0037] 1. Synthesis of Boc-GF-OMe
[0038]At room temperature, Boc-Gly-OH (10.0mmol) was dissolved in tetrahydrofuran, under ice-cooling, 1-hydroxybenzotriazole (10.0mmol) and 1-ethyl-(3-dimethylaminopropyl) carbonyl Diimine hydrochloride (10.0 mmol), reacted for 30 minutes. Continue to add HCl·Phe-OMe (10.0 mmol), adjust the pH value at 8-9 with N,N-diisopropylethylamine, and stir overnight at room temperature. After the reaction was completed, the solvent was spin-dried by a rotary evaporator, and the obtained oil was dissolved in 30 mL of ethyl acetate and transferred to a 100 mL separatory funnel. The ethyl acetate layer was washed with 50 mL of 5% NaHCO 3 Extract and wash three times, 50mL 5% NaHSO 4 Solution extraction and washing three times, 50mL saturated NaCl solution extraction and washing three times, 50mL 5% NaHCO 3 Extract and wash three times, extract and wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com