Polypeptide targeting to inhibit Wnt/beta-catenin signal activity and application thereof
A specific, peptide derivative technology, applied in the biological field, can solve the problems of complex Wnt/β-catenin signaling pathway regulation mechanism, slow drug development process, lack of inhibition of β-catenin transcriptional activation activity, etc. Safe, less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
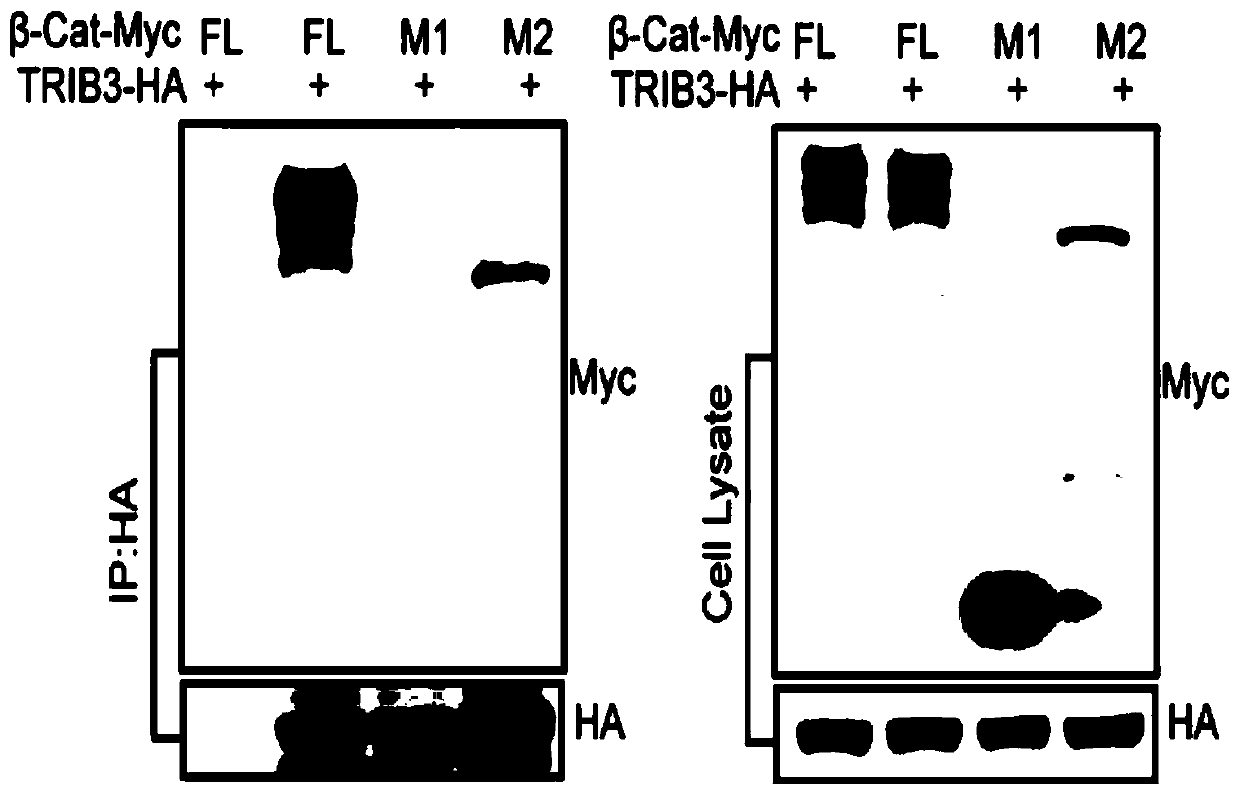
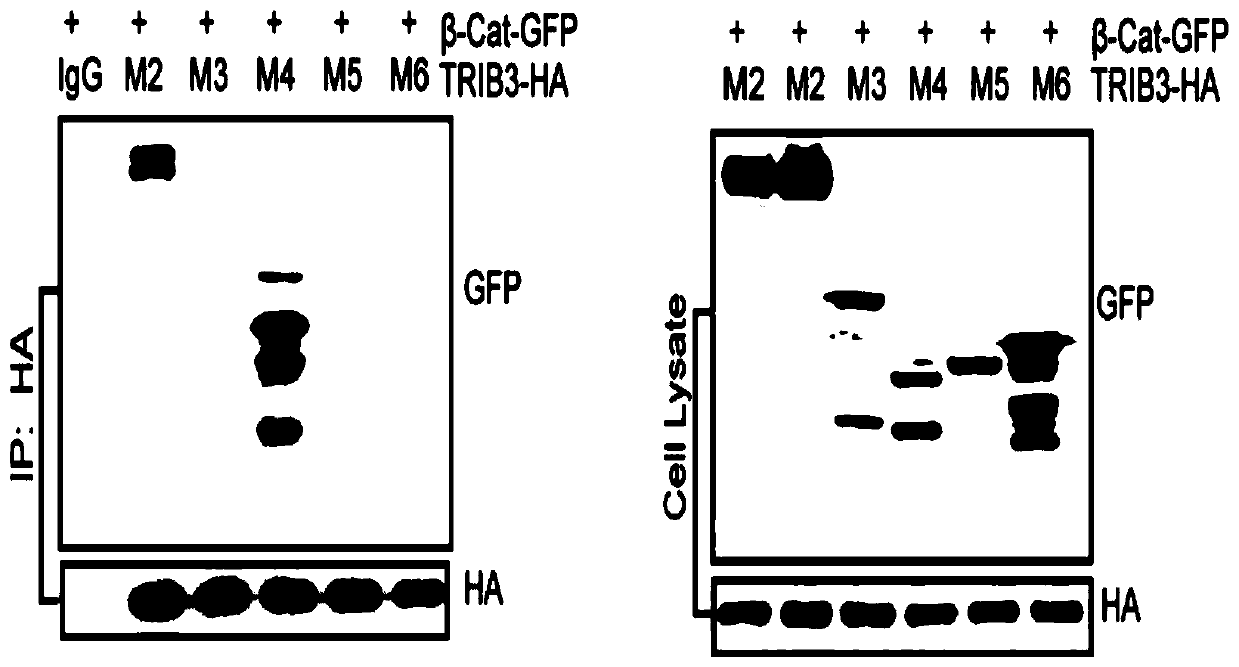
[0026] Example 1: Co-immunoprecipitation method confirms the domain that interacts with TRIB3 in β-catenin.
[0027] Co-immunoprecipitation reagents are as follows:
[0028] Lysis solution A: 0.6057g Tris base, 1.7532g NaCl, 0.1017g MgCl2 6H2O, 0.0742g EDTA, 10mL glycerin, 10mL 10% NP40, add deionized water to 150mL, adjust the pH value to 7.6 with HCl, and adjust the volume to 191mL , mixed thoroughly, filtered through a 0.45 μm membrane filter, and stored at 4°C.
[0029]Lysis Solution B: 200 μL 2M β-glycerol phosphate, 4 mL 2.5M NaF, 2 mL 8 mM NaVO3, 2 mL 100 mMPMSF, 200 μL 1M DTT, 200 μL each of 1 mg / mL Leu, Pep and Apr, total volume 9 mL. The mother liquor was stored at -20°C. Before use, thaw the mother liquor of each component in liquid B, add it to liquid A according to the above composition ratio and mix well. Protein A / GPlus-Agarose was purchased from Santa Cruz, USA. The specific operation steps are as follows:
[0030] First, the full-length gene of β-catenin ...
Embodiment 2
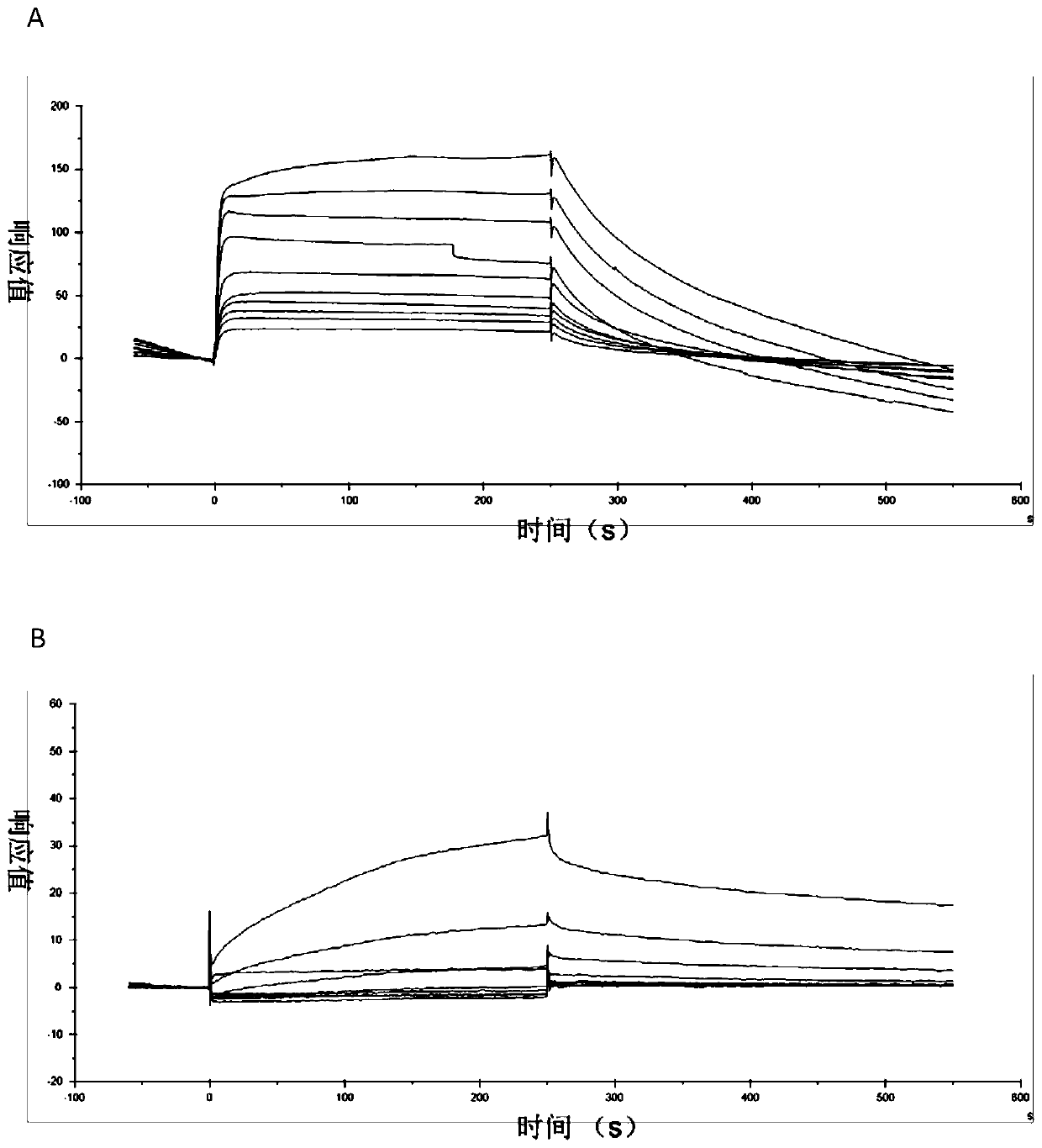
[0032] Example 2: Using surface plasmon resonance to screen peptides that bind to TRIB3 protein.
[0033] The M4 domain, which interacts with TRIB3, was analyzed using the I-TASSER website, and it was found that it contained 8 α-helical peptides. The peptide was synthesized with a peptide solid-phase synthesizer, and this process was carried out by Beijing Saibaisheng Gene Co., Ltd. EXAMPLES The entire screening process was carried out in a surface plasmon resonance instrument Biacore T200. The screening method is as follows: 1. The purified protein TRIB3 (purchased from Beijing Yiqiao Shenzhou Co., Ltd.) is coupled to the CM5 chip (purchased from GE Company) through amino groups, and the unbound protein is washed away at a flow rate of 10 μL / min, and the chip is equilibrated. Surface for 2 hours.
[0034] 200 μL of different concentrations of polypeptide fragments (200, 100, 50, 25, 12.5 nM) were automatically injected, and the whole process was carried out at 25°C. The buf...
Embodiment 3
[0037] Example 3 The ELISA method verifies the binding of the peptide ARM7 to the protein TRIB3.
[0038] The specific operation steps are as follows:
[0039] 1. Dilute human TRIB3 protein and bovine serum albumin (BSA) to 10 μg / ml with PBS, add 100 μl to each well, and coat 96-well ELISA plate overnight at 4°C.
[0040] 2. Wash three times with PBS containing 0.1% Tween-20. The plates were coated with 200 μl of blocking solution (10% bovine serum in PBS), and coated at 37° C. for 2 hours.
[0041] 3. Pour off the coating solution, add 200 μl of 1 μg / ml polypeptide ARM7 solution correspondingly, set up positive control wells at the same time, add 200 μl of 1 μg / ml β-catenin protein solution, and incubate at 37°C for 1 hour.
[0042] 4. Wash five times with PBS containing 0.1% Tween-20. Add 100 μl anti-TRIB3 monoclonal antibody diluted with blocking solution 1:4000 to each well, and incubate at room temperature for 1 h.
[0043] 5. Wash six times with PBS containing 0.1% T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com