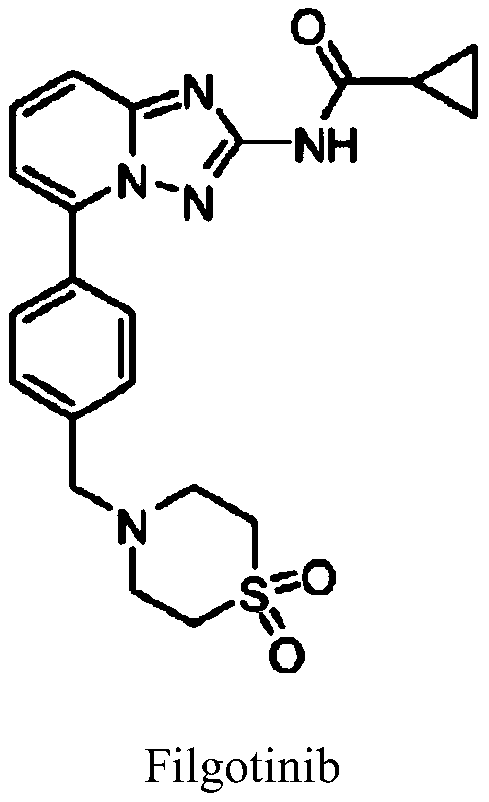
Synthesis method of JAK1 inhibitor Filgotinib
A compound and catalyst technology, applied in the field of synthesis of JAK1 inhibitor Filgotinib, can solve the problems of ineffective correction of rheumatoid arthritis and osteoarthritis diseases, and achieve the effects of simple reaction operation, high yield and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
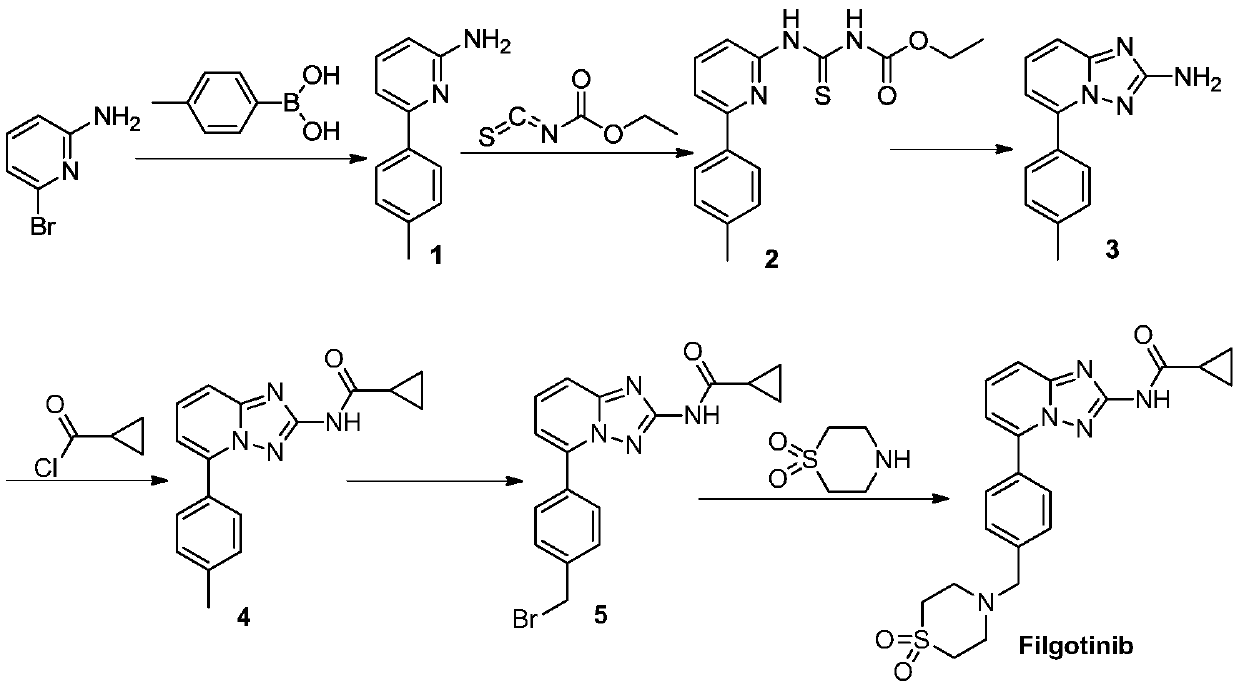
[0062] Embodiment 1, the synthesis of compound Filgotinib
[0063] According to the following synthetic route, compound Filgotinib is prepared:
[0064]
[0065] 1, Synthesis of 6-(4-methylphenyl)-2-aminopyridine (compound 1)
[0066] Add 2-amino-6-bromopyridine (50g, 0.29mol), p-tolylboronic acid (47g, 0.35mol), 1,4-dioxane (750ml) and water (250ml) into the reaction flask, Potassium acetate (85 g, 0.87 mol) and [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (Pd(dppf)Cl) were added 2 , 12.7g, 0.0174mol). Nitrogen was introduced, heated to reflux for 13 hours, and the reaction was monitored by TLC. Cool down to room temperature, add 2N hydrochloric acid to PH>3, precipitate solid, filter, dissolve the filter cake with 2N aqueous sodium hydroxide solution, extract with ethyl acetate (300mlX3), concentrate the organic phase to obtain the product 6-(4-methylphenyl )-2-aminopyridine (compound 1) 144.7g, yield 83.6%.
[0067] 2. Synthesis of [6-(4-methylphenyl)p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com