High-efficiency carbon nanosheet supported fuel cell positive electrode material and preparation method and application thereof
A carbon nanosheet, fuel cell technology, applied in battery electrodes, nanotechnology for materials and surface science, nanotechnology, etc., can solve problems such as low efficiency and poor stability, and achieve excellent stability and excellent oxygen. The effect of reducing performance, simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
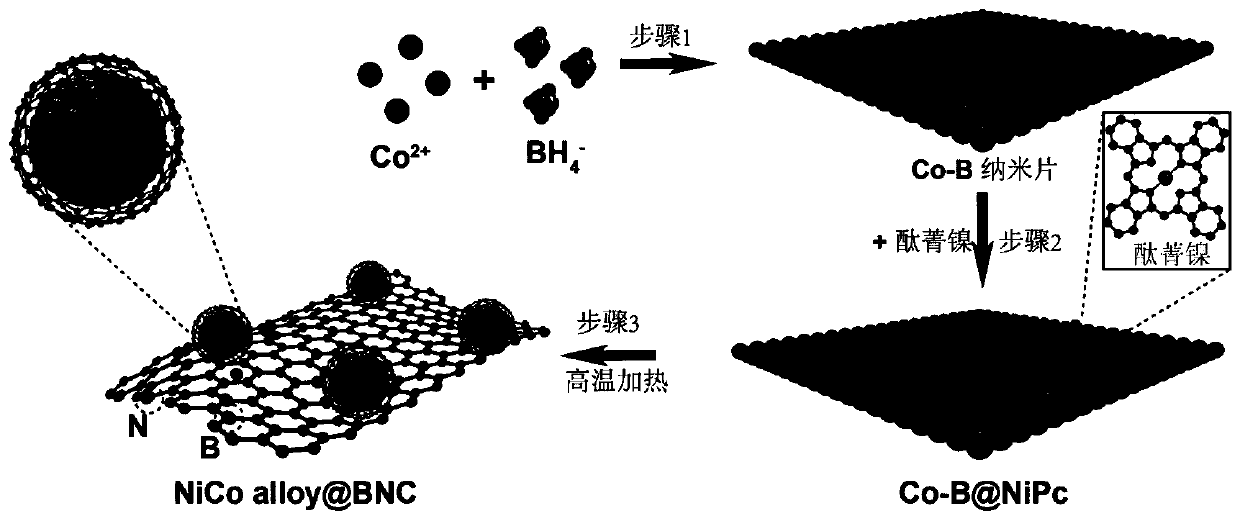
[0040] The formation mechanism of amorphous Co-B nanosheets is as follows: loaded with 0.1M Co(NO 3 ) 2 ·6H 2 The three-necked flask of the O solution was filled with N in an ice-water bath. 2 Stir continuously for more than 30min under the condition, so that the Co ions are evenly dispersed in the solution. Afterwards, place 0.5M NaBH in an ice-water bath 4 Alkaline solution (0.1M KOH) was added dropwise to Co(NO 3 ) 2 In the solution, the period is required to be carried out under the condition of anaerobic and vigorous stirring, as shown in formula 1.
[0041] co 2+ +BH 4 - +H 2 O→Co-B+H 2 +B(OH) 3 (1)
[0042] After about 30 minutes of reaction, the generation of black substance can be clearly observed. Finally, after washing with water and washing with alcohol three times each, the sample obtained by centrifugation was dried overnight under vacuum. Its mechanism diagram is as follows figure 1 As shown, its topography is shown in diagram 2-1 shown.
Embodiment 2
[0044] As described in Example 1, the obtained Co-B and nickel phthalocyanine powders were fully dispersed in the ethanol solution under the condition of ultrasound for 1 h at a mass ratio of 1:1; Stir vigorously for 24 hours; finally, wash with water and alcohol for 3 times, then centrifuge, and dry the obtained sample under vacuum overnight.
Embodiment 3
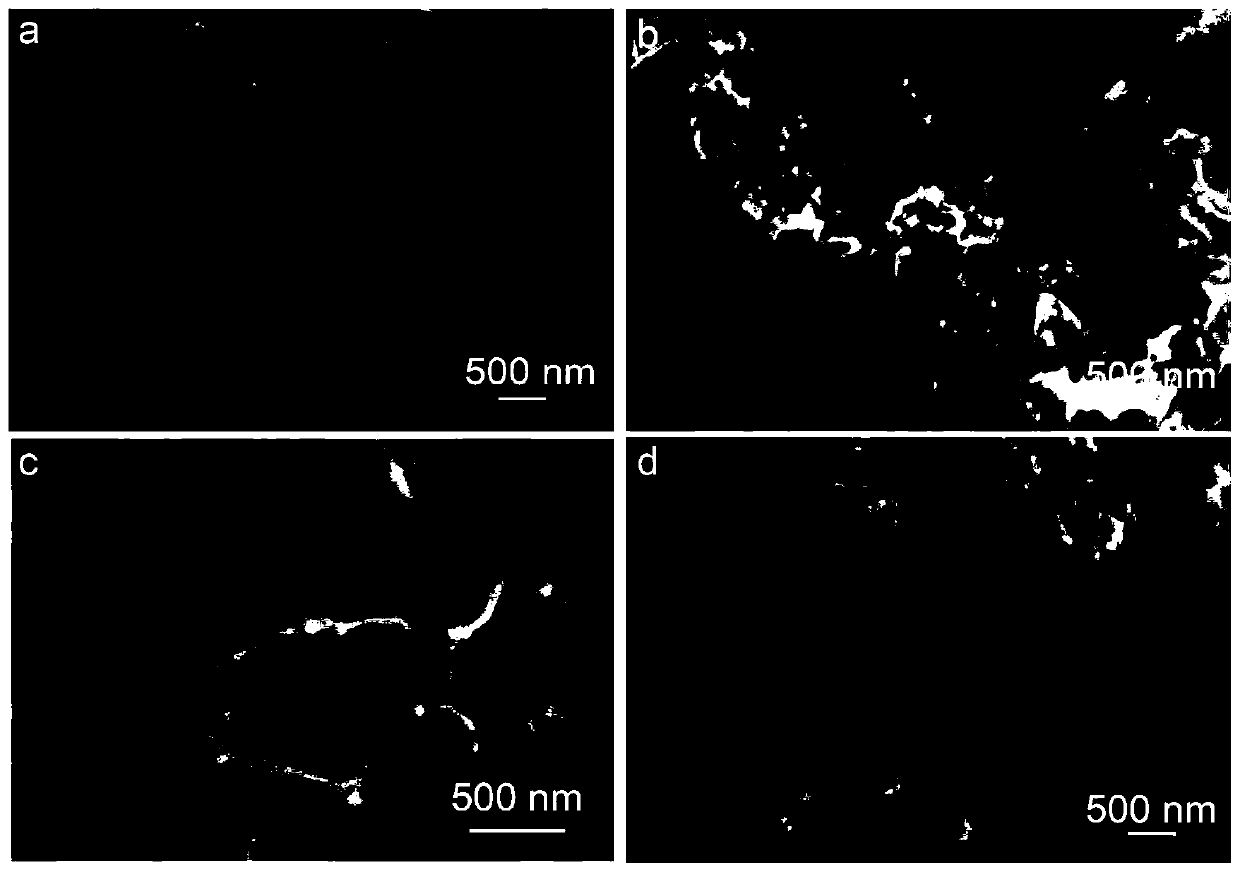
[0046] The resulting Co-B and nickel phthalocyanine mixture was placed in the middle of a CVD quartz tube as described in Example 2. First, the air pressure in the tube is pumped down to below 5pa by the action of the vacuum pump; at this time, the argon pressure reducing valve is opened, and 100 sccm of argon is continuously passed into the tube, and the two processes of vacuuming and argon are alternately carried out at least 3 times to remove the air inside. Finally, start the heating program, the heating rate is less than 2°C / min, and the heating temperatures are respectively 400°C, 600°C, 800°C, and 1000°C. The morphologies of catalysts obtained under different temperature conditions are as follows: Figure 2-2 As shown, the special structure supported by uniform alloy nanoparticles can only be formed when the temperature is higher than 400 °C and lower than 1000 °C. XRD patterns of samples at different temperatures Figure 2-3 As shown, the synthesis temperature of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com