Preparation method and application of hydrogen peroxide decomposed manganese oxide catalysts
A manganese oxide and hydrogen peroxide technology, which is applied in the field of metal oxide catalyst preparation, can solve the problems of inaccurate attribute identification, great differences in the characteristics of manganese dioxide products, inconsistent effects of hydrogen peroxide, etc., and achieves low production cost. , the effect of good application and promotion value, simple and easy preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] In this example, a δ-MnO 2 The preparation method of catalyst, concrete steps are as follows:
[0048] a1. Weigh 0.5g potassium permanganate and 0.2g manganese sulfate monohydrate into 50mL of distilled water, stir fully at room temperature to fully dissolve potassium permanganate and manganese sulfate, and prepare potassium permanganate and manganese sulfate mixed solution;
[0049] a2. Move the mixed solution prepared according to step a1 to a 50mL polytetrafluoroethylene reactor, put the reactor into an oven at 140°C, react for 1 hour, and cool naturally to room temperature to obtain the reaction product;
[0050] a3. Wash the reaction product obtained in step a2 with twice distilled water for 3 to 4 times to obtain the washings; dry the washings at 60°C for 12 hours to obtain the final product δ-MnO 2 .
[0051] Experimental test analysis:
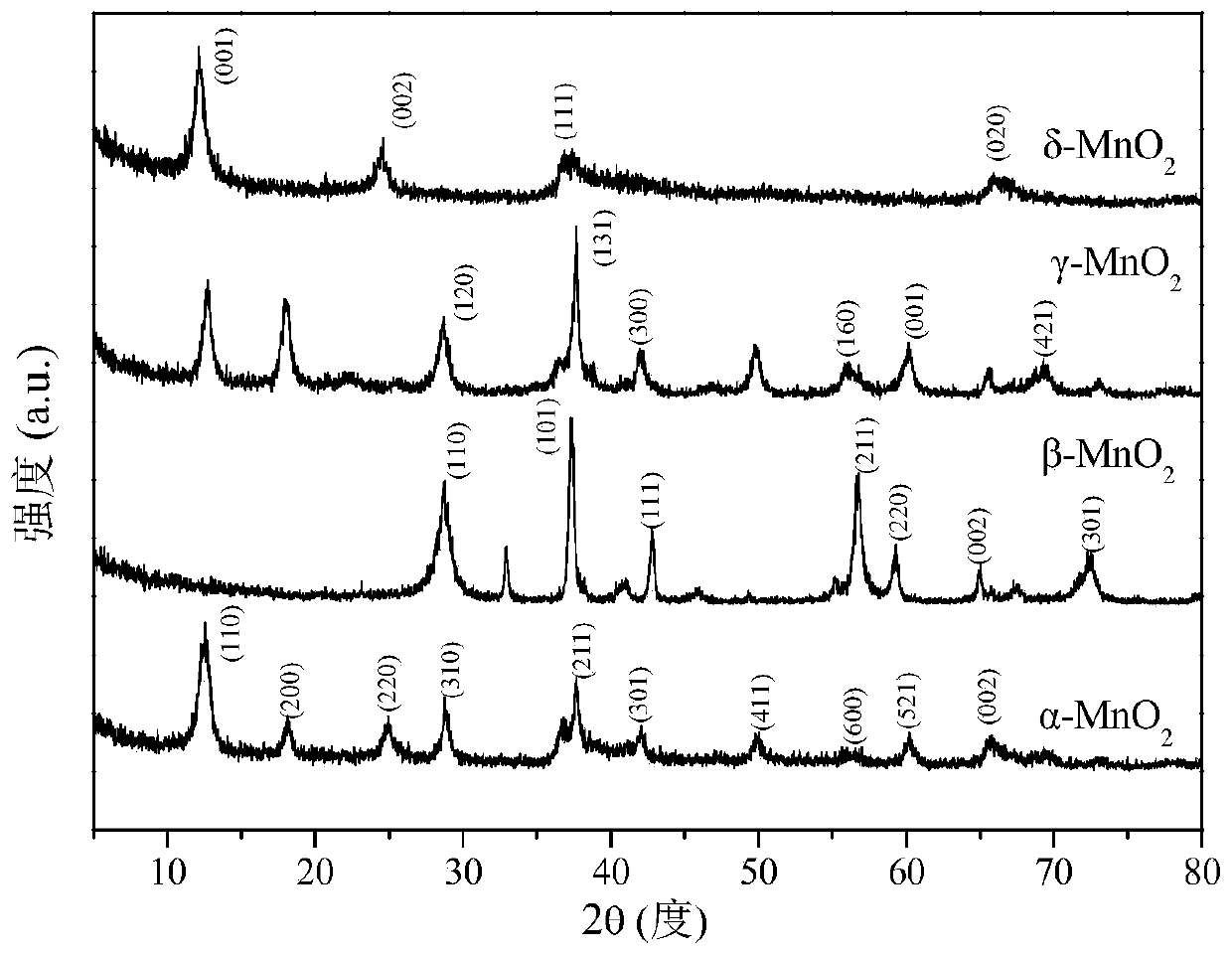
[0052] The δ-MnO prepared in this example 2 The catalyst was analyzed by X-ray diffraction and obtained as figure 1 The ...
Embodiment 2
[0054] This embodiment is basically the same as Embodiment 1, especially in that:
[0055] In this example, an α-MnO 2 The preparation method of catalyst, concrete steps are as follows:
[0056] b1. Weigh 0.5g of potassium permanganate and 0.2g of manganese sulfate monohydrate into 50mL of distilled water, stir fully at room temperature to fully dissolve potassium permanganate and manganese sulfate, and prepare a mixture containing potassium permanganate and manganese sulfate mixed solution;
[0057] b2. Transfer the mixed solution prepared according to step b1 into a 50mL polytetrafluoroethylene reactor, put the reactor into an oven at 140°C, react for 2 hours, and naturally cool to room temperature to obtain the reaction product;
[0058] b3. Wash the reaction product obtained in step b2 with double distilled water for 3 to 4 times to obtain the washings; dry the washings at 60°C for 12 hours to obtain the final product α-MnO 2 .
[0059] Experimental test analysis:
[...
Embodiment 3
[0062] This embodiment is basically the same as the previous embodiment, and the special features are:
[0063] In this example, a β-MnO 2 The preparation method of catalyst, concrete steps are as follows:
[0064] c1. Measure 40mL of 0.5mol / L manganese nitrate tetrahydrate solution and pour it into a 100mL ceramic crucible;
[0065] c2. Place the ceramic crucible filled with the manganese nitrate solution in the step c1 in a muffle furnace and bake at 400°C for 4 hours, then cool to room temperature, and grind to obtain the product brown-black β-MnO 2 .
[0066] Experimental test analysis:
[0067] The β-MnO prepared in this embodiment 2 The catalyst was analyzed by X-ray diffraction and obtained as figure 1 The results shown. It can be seen from the figure that β-MnO 2 There are obvious diffraction peaks at 2θ=28.64°, 37.41°, 42.71°, 56.81°, 59.25°, 65.12° and 72.53°, which correspond to (110), (101), (111), (211 ), (220), (002) and (301) crystal faces, which are com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com