4-cyanobenzyloxy-4'-cyanophenyl ether preparation method
A technology of cyanophenyl ether and cyanobenzyloxy, applied in the field of organic compound preparation, can solve the problems of low yield, harsh conditions and the like, and achieve the effects of high yield, easy product and simple operation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
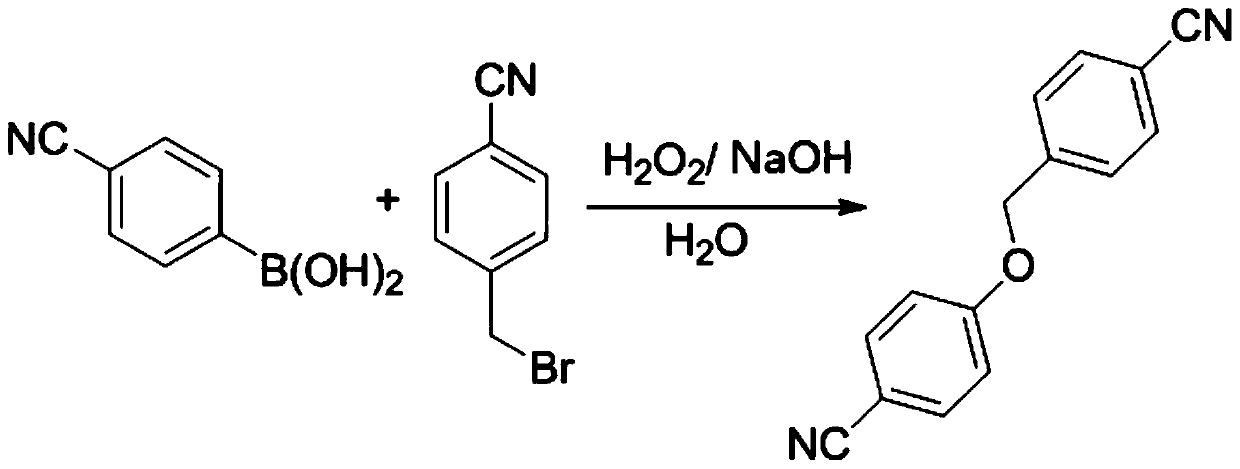
[0016] The invention provides a kind of preparation method of 4-cyanobenzyloxy-4'-cyanophenyl ether, comprising the following steps:
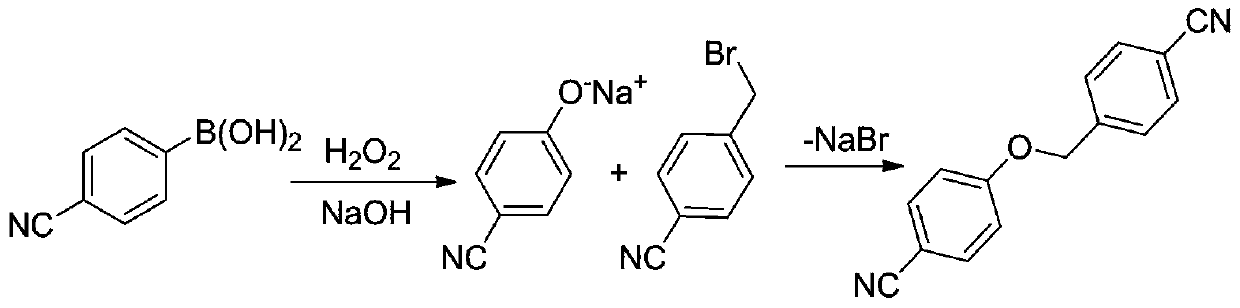
[0017] Mix and heat p-cyanobenzyl bromide, 4-cyanophenylboronic acid, sodium hydroxide, hydrogen peroxide solution and water for oxidation and nucleophilic substitution reaction to obtain 4-cyanobenzyloxy-4'-cyanobenzene base ether.
[0018] In the present invention, p-cyanobenzyl bromide, 4-cyanophenylboronic acid, sodium hydroxide, hydrogen peroxide solution and water are mixed and heated, and after oxidation and nucleophilic substitution reaction, 4-cyanobenzyloxy-4'-cyanide is obtained phenyl ether. In the present invention, the dosage ratio of the p-cyanobenzyl bromide, 4-cyanophenylboronic acid, sodium hydroxide, hydrogen peroxide solution and water is preferably 0.1960~1.960g:0.1761~1.763g:0.0640~0.640g: 0.1-1.0mL: 6-60mL, more preferably 0.1960-0.980g: 0.1761-0.8805g: 0.0640-0.320g: 0.1-0.5mL: 6-30mL, more preferably 0.1960g: 0.1761g:...
Embodiment 1
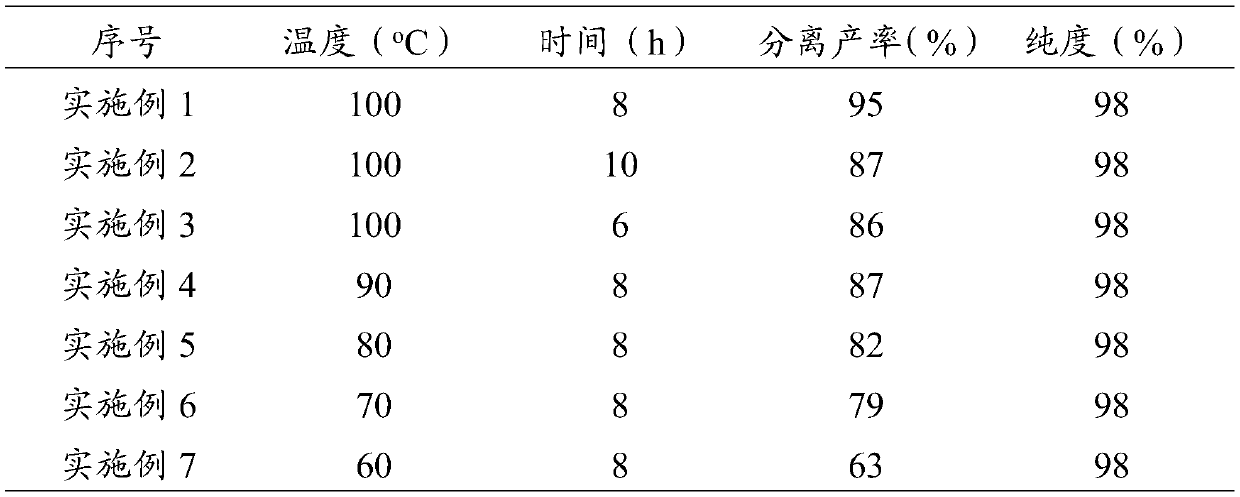
[0030] Add 0.1960g (1mmol) of p-cyanobenzyl bromide, 0.1763g (1.2mmol) of 4-cyanophenylboronic acid, 0.0640g (1.6mmol) of NaOH, H 2 O 6mL, add H under stirring 2 o 2 0.2mL (30%), heated and stirred in an oil bath at 100°C for 8h, the precipitated product precipitated, filtered while hot, dissolved the precipitated product obtained by filtration with ethyl acetate, filtered to remove insoluble matter, added anhydrous sodium sulfate to dry, filtered The desiccant was removed, and the product was crystallized in ethyl acetate to obtain 0.2226 g of pure product with a yield of 95% and a purity of 98%.
[0031] The melting point of the prepared 4-cyanobenzyloxy-4'-cyanophenyl ether was tested by using the automatic melting point instrument of Jinan Haineng Instrument Co., Ltd. model Haineng MP120, and the melting point was 167 ~168°C.
[0032] Adopt the model of German Bruker company to be that the nuclear magnetic resonance instrument of Bruker Avance III (400MHz) type is teste...
Embodiment 2
[0034]According to the method of Example 1 to prepare 4-cyanobenzyloxy-4'-cyanophenyl ether, the difference from Example 1 is that the reaction time is different, and the reaction time in this example is 10h. See Table 1 for the purity and yield of the prepared 4-cyanobenzyloxy-4'-cyanophenyl ether.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com