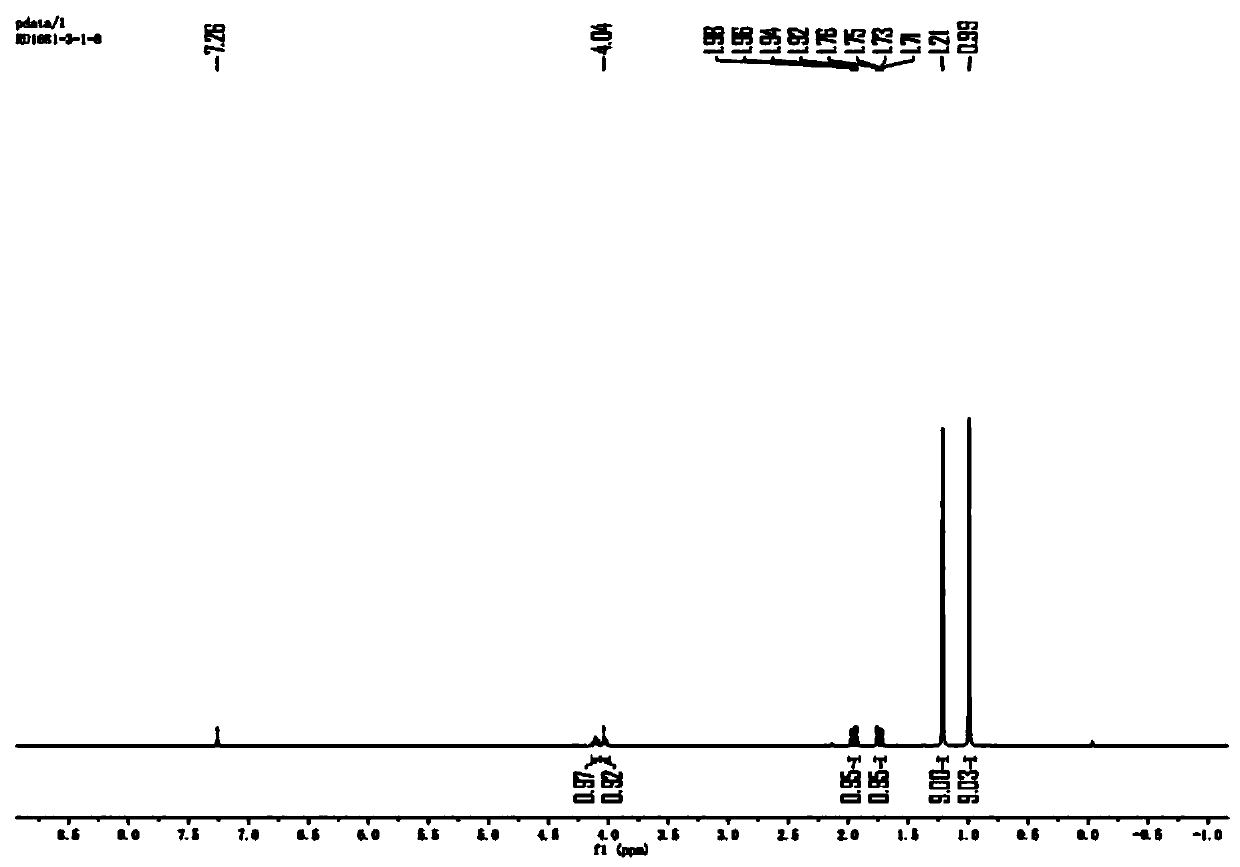
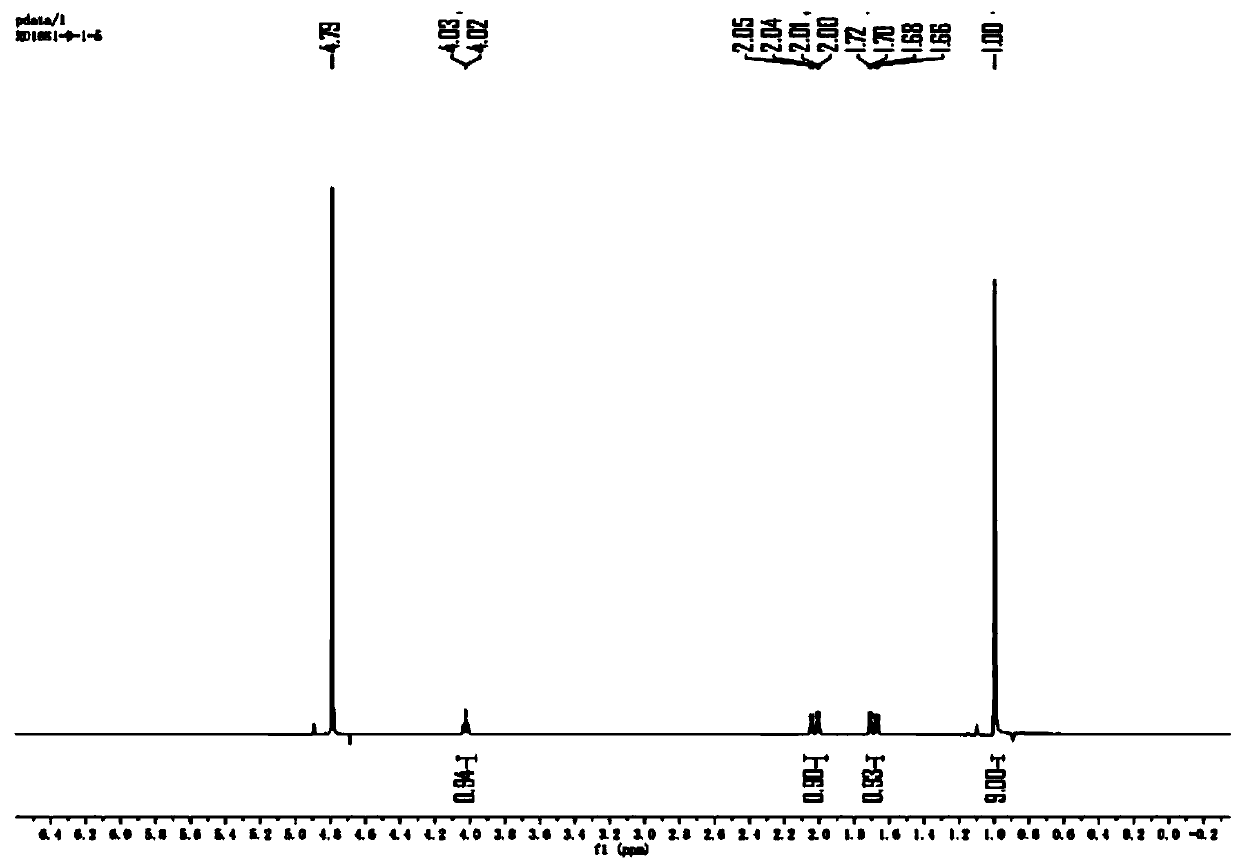
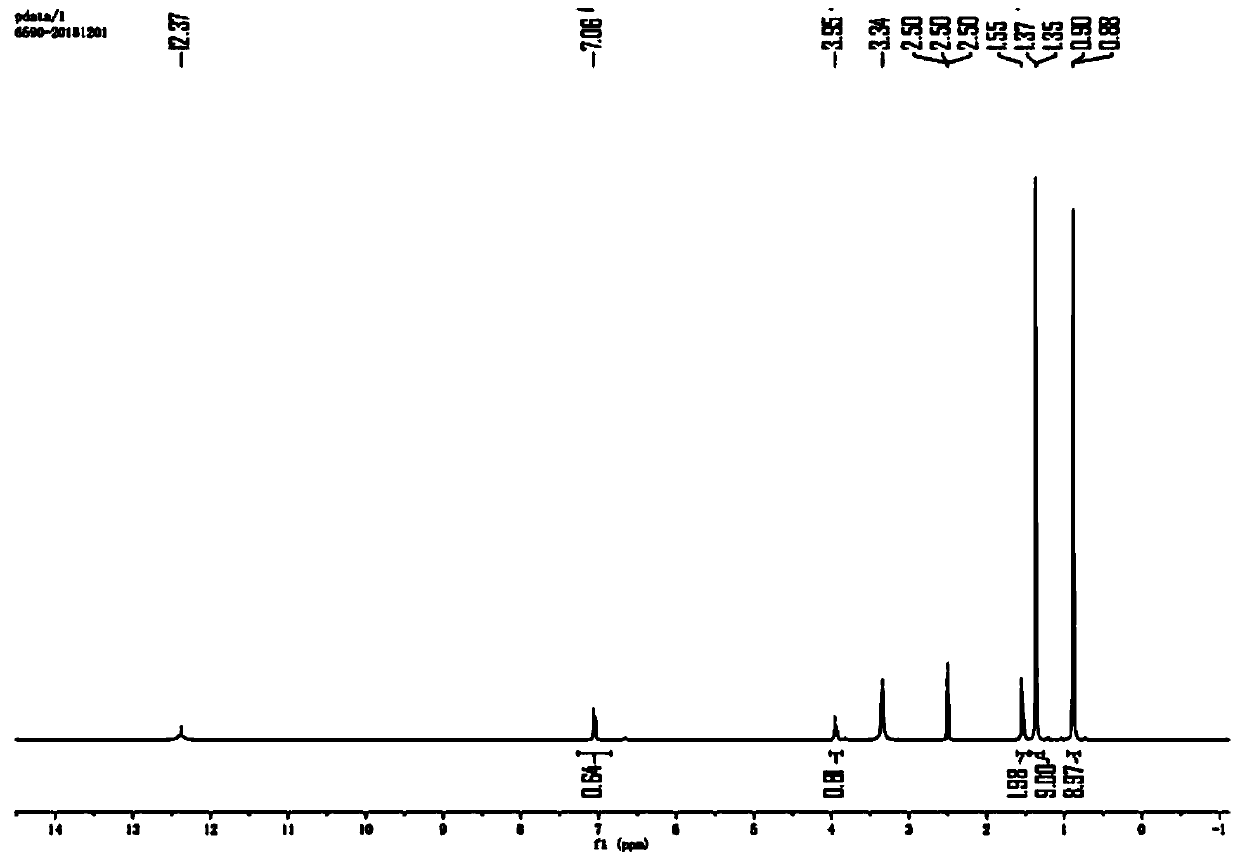
Preparation method of N-Boc-(R)-2-amino-4, 4-dimethylvaleric acid
A technology of dimethylvaleric acid and dimethylvaleric hydrochloride is applied in the preparation of carbamate derivatives, the preparation of organic compounds, the preparation of cyanide reactions, etc., and can solve the problem of low product yield and expensive enzyme catalysts and other problems, to achieve the effect of cheap and easy-to-obtain raw materials, low cost, and safe product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A preparation method of N-Boc-(R)-2-amino-4,4-dimethylvaleric acid, comprising the following steps:
[0032] S1. Under nitrogen atmosphere, dissolve 3,3-dimethylbutyraldehyde (70g, 1.0eq) in 42mL of dichloromethane and add (R)-(+)-tert-butylsulfinamide (93.2g, 1.1eq ) and anhydrous copper sulfate (178.4g, 1.6eq), then stirred and reacted at 20°C for 12h, after the reaction was completed, filtered and concentrated to obtain (R,E)-N-(3,3-dimethyl Butylene)-2-methylpropane-2-sulfinamide, yield 76%;
[0033] S2. The (R,E)-N-(3,3-dimethylbutylene)-2-methylpropane-2-sulfinamide (107g, 1.0eq) was dissolved in n-hexane (749mL) and Add cesium fluoride (84g, 1.05eq), add trimethylsilyl cyanide (164.4g, 3.0eq) dropwise to the system under an inert atmosphere at -5°C, after the addition is complete, react at -5°C for 60h , add 40mL of saturated ammonium chloride to the system to quench, extract with ethyl acetate, dry the organic phase over anhydrous sodium sulfate, filter, conce...
Embodiment 2
[0037] A preparation method of N-Boc-(R)-2-amino-4,4-dimethylvaleric acid, comprising the following steps:
[0038] S1. Under a nitrogen atmosphere, dissolve 3,3-dimethylbutyraldehyde (70g, 1.0eq) in 50mL of dichloromethane and add (R)-(+)-tert-butylsulfinamide (93.2g, 1.0eq ) and anhydrous copper sulfate (33.45g, 0.3eq), then stirred and reacted at 20°C for 12h, after the reaction was completed, filtered and concentrated to obtain (R,E)-N-(3,3-dimethyl Butylene)-2-methylpropane-2-sulfinamide, yield 35%;
[0039]S2. The (R,E)-N-(3,3-dimethylbutylene)-2-methylpropane-2-sulfinamide (107g, 1.0eq) was dissolved in n-heptane (749mL) And add cesium fluoride (84g, 1.05eq), in an inert atmosphere and at -5°C, add trimethylsilyl cyanide (164.4g, 3.0eq) dropwise to the system, after the dropwise addition, react at 0°C for 72h , add 40mL of saturated ammonium chloride to the system to quench, extract with ethyl acetate, dry the organic phase over anhydrous sodium sulfate, filter, conce...
Embodiment 3
[0043] A preparation method of N-Boc-(R)-2-amino-4,4-dimethylvaleric acid, comprising the following steps:
[0044] S1. Under a nitrogen atmosphere, dissolve 3,3-dimethylbutyraldehyde (70g, 1.0eq) in 60mL of tetrahydrofuran and add (R)-(+)-tert-butylsulfinamide (101.7g, 1.2eq) and Anhydrous copper sulfate (278.75g, 2.5eq), then stirred and reacted at 20°C for 12h, after the reaction was completed, filtered and concentrated to obtain (R,E)-N-(3,3-dimethylbutylene )-2-methylpropane-2-sulfinamide, yield 60%;
[0045] S2. The (R,E)-N-(3,3-dimethylbutylene)-2-methylpropane-2-sulfinamide (107g, 1.0eq) was dissolved in n-heptane (749mL) And add cesium fluoride (84g, 1.05eq), in an inert atmosphere and at -5°C, add trimethylsilyl cyanide (164.4g, 3.0eq) dropwise to the system, after the dropwise addition, react at -5°C for 60h Finally, add 40 mL of saturated ammonium chloride to the system to quench, extract with ethyl acetate, dry the organic phase over anhydrous sodium sulfate, fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com