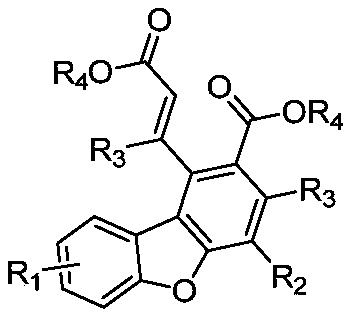
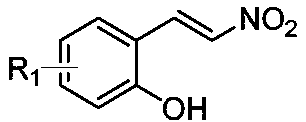
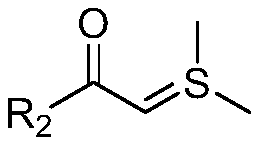
Dibenzofuran acrylate compound and preparation method therefor
A technology of furan acrylates and compounds, which is applied in the field of dibenzofuran acrylate compounds and their preparation, can solve problems such as the inability to meet the needs of direct synthesis of structurally diverse multifunctional group dibenzofuran derivatives, and achieve substrate application The effect of wide range of properties, high reaction yield, and simple and easy synthetic method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Dimethyl(Z)-8-chloro-1-(1,4-dimethoxy-1,4-dioxobut-2-en-2-yl)-4-(p-toluene Yl)dibenzo[b,d]furan-2,3-dicarboxylic acid
[0066]
[0067] Combine (E)-4-chloro-2-(2-nitroalkene)phenol (1.0mmol, 1.0 equivalent) and 2-(dimethyl-λ 4 -Sulfylene)-1-(p-tolyl)ethane-1-one (1.0 mmol, 1.0 equivalent) was dissolved in 8 mL of acetonitrile, and stirred at 35°C for half an hour. Then, dimethyl dimethylate (2.1 mmol, 2.1 equivalent) and potassium carbonate (1.0 mmol, 1.0 equivalent) were added to the above system, and stirring was continued for 12 hours at 35°C. After the reaction was completed, the reaction system was diluted with 20 mL of ethyl acetate. Then it was washed once with 5 mL of water, separated into the ethyl acetate layer and concentrated under reduced pressure. The obtained crude product was separated and purified by silica gel column chromatography (petroleum ether: ethyl acetate = 7:1) to obtain a white powder with a yield of 78%.
[0068] White solid, yield ...
Embodiment 2
[0082] Example 2: Dimethyl (Z)-8-chloro-1-(1,4-dimethoxy-1,4-dioxobut-2-en-2-yl)-4-phenyldi Benzo[b,d]furan-2,3-dicarboxylic acid
[0083]
[0084] The synthesis steps are the same as in Example 1, except that 2-(dimethyl-λ 4 -Sulfylene)-1-(p-tolyl)ethane-1-one replaced with 2-(dimethyl-λ 4 -Sulfylene)-1-phenylethane-1-one to obtain a white solid with a yield of 74%.
[0085] White solid, yield 74%. Melting point: 115.1-116.2°C. 1 H NMR(500MHz, CDCl 3 )δ8.10(d,J=2.2Hz,1H), 7.53-7.48(m,5H), 7.484-7.473(m,2H), 6.43(s,1H), 3.96(s,3H), 3.88(s , 3H), 3.79 (s, 3H), 3.62 (s, 3H). 13 C NMR(125MHz, CDCl 3 )δ167.93,166.50,165.78,164.82,155.71,154.93,137.36,133.66,132.76,130.78,130.16,129.46,129.40,128.94,128.85,128.50,125.97,125.55,124.15,123.66,122.93,113.28,53.00,52.72. ,52.62. HRMS(ESI):m / z calcd for[2M+Na] + :1095.1640,found:1095.1639.
Embodiment 3
[0086] Example 3: Dimethyl (Z)-8-chloro-1-(1,4-dimethoxy-1,4-dioxobut-2-en-2-yl)-4-(3- Methoxyphenyl) dibenzo[b,d]furan-2,3-dicarboxylic acid
[0087]
[0088] The synthesis steps are the same as in Example 1, except that 2-(dimethyl-λ 4 -Sulfylene)-1-(p-tolyl)ethane-1-one replaced with 2-(dimethyl-λ 4 -Sulfylene)-1-(3-methoxyphenyl)ethane-1-one to obtain a white solid with a yield of 76%.
[0089] White solid, yield 76%. Melting point: 139.8-141.4°C. 1 H NMR(500MHz, CDCl 3 )δ8.10(d,J=1.2Hz,1H),7.48(dd,J=7.2,5.1Hz,2H),7.42(t,J=7.9Hz,1H),7.11–6.99(m,3H), 6.42 (s, 1H), 3.96 (s, 3H), 3.88 (s, 3H), 3.85 (s, 3H), 3.78 (s, 3H), 3.65 (s, 3H). 13 C NMR(125MHz, CDCl 3 )δ167.91,166.46,165.76,164.79,159.48,155.70,154.86,137.35,133.97,133.61,130.78,130.19,129.57,129.41,128.95,125.79,125.54,124.13,123.68,122.92,121.83,115.01,114.55.36,113. ,52.98,52.80,52.70,52.68.HRMS(ESI):m / z calcd for[2M+Na] + :1155.1852,found:1155.1856.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com