Rapid preparing method of Zr-based electrochemical amorphous alloy
An amorphous alloy, electrochemical technology, applied in the direction of optics, process efficiency improvement, photography technology, etc., can solve the problems of reduction potential difference, complex stability of plating solution, low-level amorphous alloy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
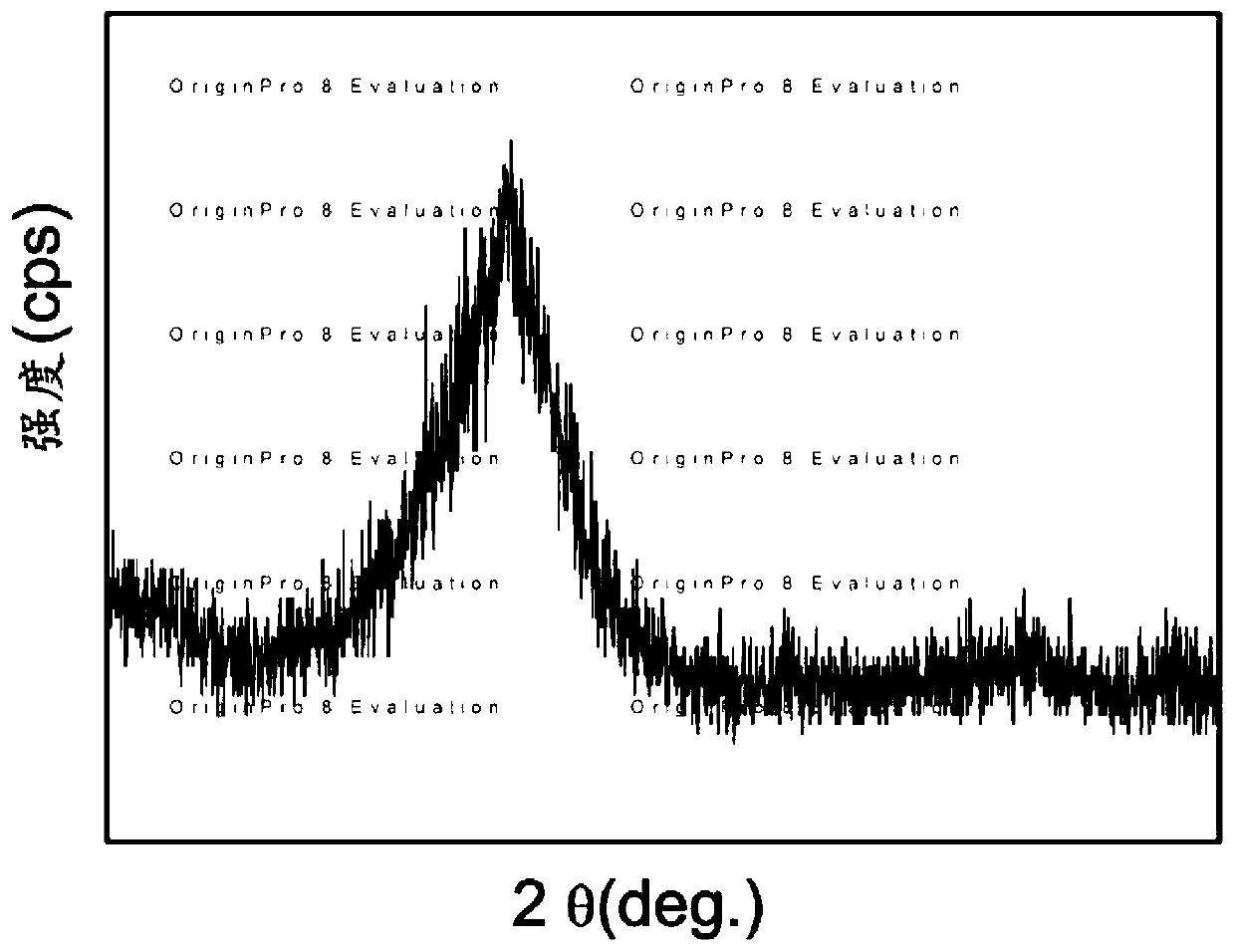
Image
Examples
Embodiment 1
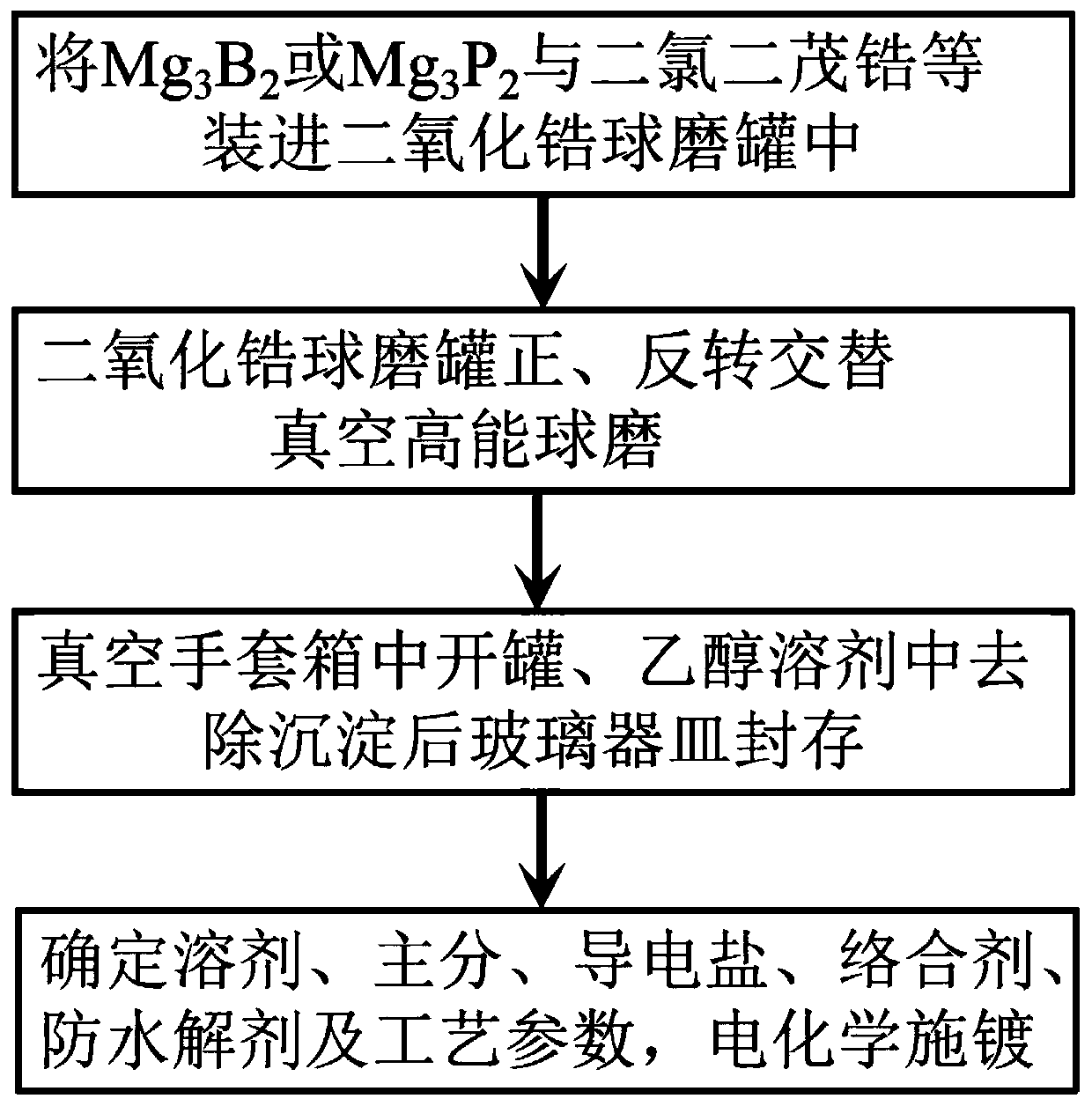
[0024] Use Mg 3 B 2 Zr-based polycation salt is produced with dicene zirconium dichloride, and Zr-based amorphous alloy (including coating) is obtained by solution ion plating on this basis. The specific steps are:
[0025] Step one, Mg 3 B 2 It is packed into a zirconium dioxide ball mill tank at a molar ratio of 1:3 to 6 with dicene zirconium dichloride. The ball-to-battery ratio is selected as 15:1. A small amount of benzene is added as an anti-adhesive or diluent and filled with argon. Airtight tank, two tanks;
[0026] Step 2: Forward rotation for 5 minutes, reverse rotation for 1 minute, rotation speed 1000r / min, ball milling time 20-40 hours;
[0027] Step 3. Open the can in a vacuum glove box, stir the ball milled product in a glass beaker with ethanol as a solvent for 5-15 minutes, and let it stand for 5-15 minutes. After removing the precipitate, the glassware is sealed for use;
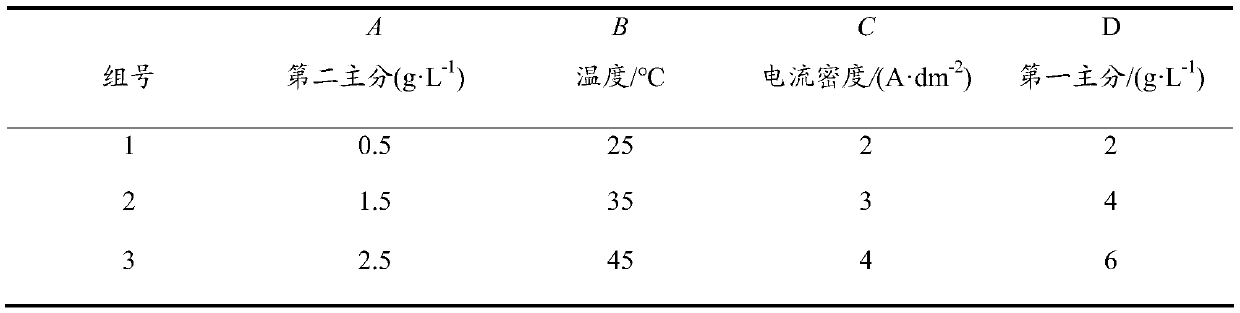
[0028] Step 4. Use ethanol as the solvent, polycation salt (1-8g / L) as the main salt, nickel a...
Embodiment 2
[0030] Use Ca 3 P 2 Zr-based polycation salt is produced with dicene zirconium dichloride, and Zr-based amorphous alloy (including coating) is obtained by solution ion plating on this basis. The specific steps are:
[0031] Step one, the Ca 3 P 2 It is packed into a zirconium dioxide ball mill tank at a molar ratio of 1:3 to 6 with dicene zirconium dichloride. The ball-to-battery ratio is selected as 15:1. A small amount of benzene is added as an anti-adhesive or diluent and filled with argon. Airtight tank, two tanks;
[0032] Step 2: Forward rotation for 5 minutes, reverse rotation for 1 minute, rotation speed 1000r / min, ball milling time 15 to 35 hours;
[0033] Step 3. Open the can in a vacuum glove box, stir the ball milled product in a glass beaker with ethanol as a solvent for 5-15 minutes, and let it stand for 5-15 minutes. After removing the precipitate, the glassware is sealed for use;
[0034] Step 4. Use ethanol as the solvent, polycation salt (2~6g / L) as the first main ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com