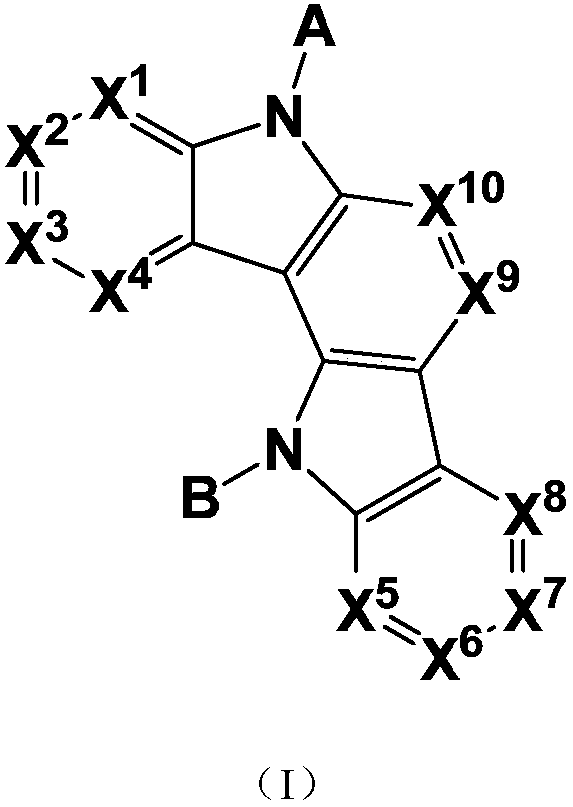
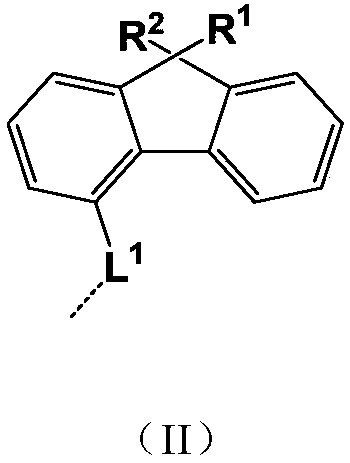
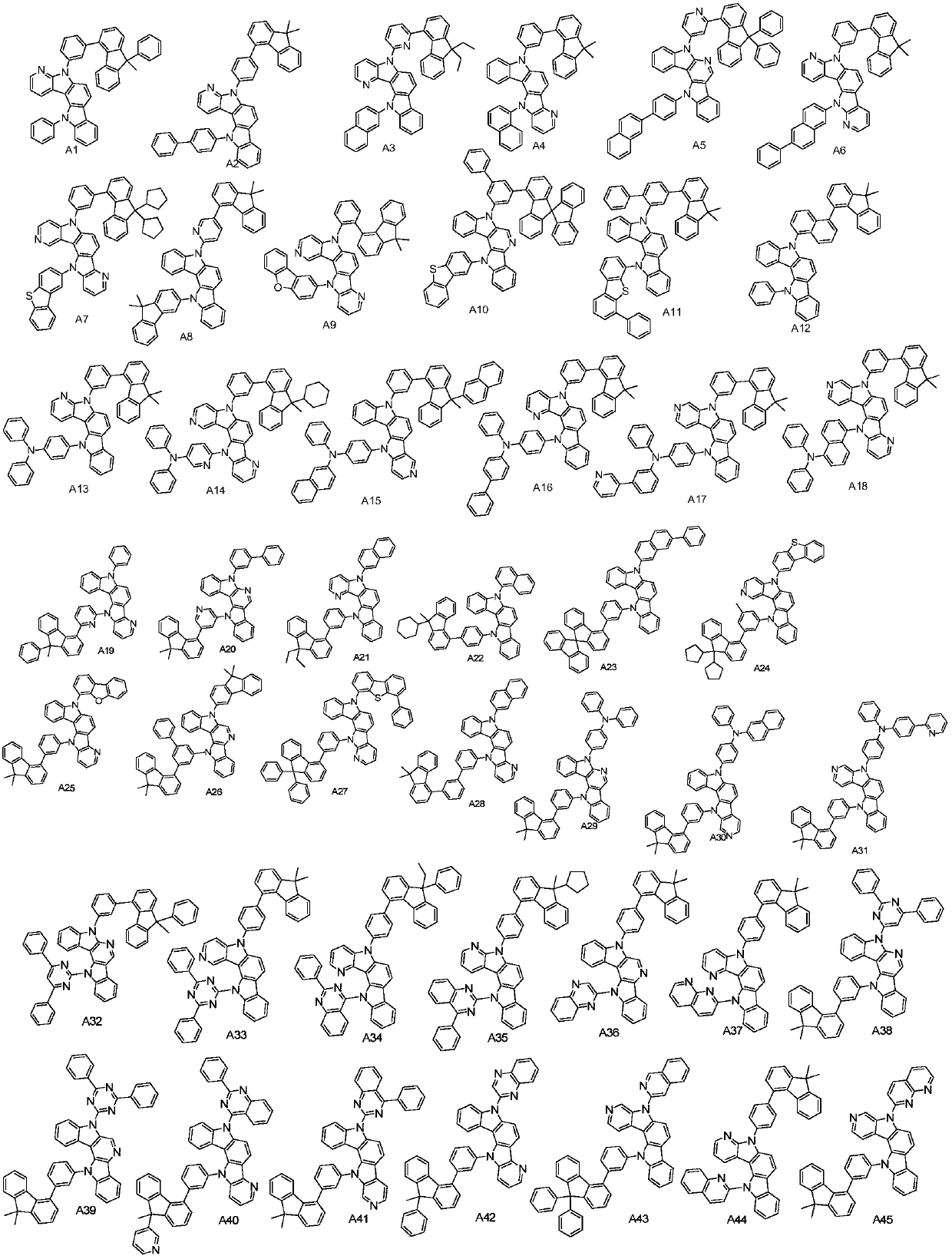
General formula compound and application thereof
A compound of general formula, selected technology, applied in the field of organic electroluminescent devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0042] Synthesis Example 1: Synthesis of Compound A2
[0043]
[0044] Under nitrogen protection, add 25.6 g (100 mmol) of indolo(3,2-A) carbazole and 38.2 g (110 mmol) of 4-(4-bromophenyl)-9,9-dimethylfluorene into the reaction flask, PD 2 (dba) 3 0.9g (0.785mmol, 0.5%), xylene 1500mL, sodium tert-butoxide 43.3g (314mmol), heated to reflux for 12h. After the reaction is complete, stop the reaction. Cool to room temperature, wash with water, concentrate the organic phase, and purify the obtained solid by recrystallization from toluene to obtain white powder M1.
[0045] Nitrogen protection, in the reaction flask, add intermediate M1 10.4g (20mmol), 4-bromobiphenyl 26.2g (110mmol), Pd2 (dba) 30.9g (0.785mmol, 0.5%), xylene 1500mL, sodium tert-butoxide 43.3g (314mmol), heated to reflux for 12h. After the reaction is complete, stop the reaction. Cool to room temperature, wash with water, concentrate the organic phase, and purify the obtained solid by recrystallization fr...
Synthetic example 2
[0046] Synthesis Example 2: Synthesis of Compound A13
[0047]
[0048] Under nitrogen protection, add 25.6 g (100 mmol) of indolo(3,2-A) carbazole and 38.2 g (110 mmol) of 4-(4-bromophenyl)-9,9-dimethylfluorene into the reaction flask, PD 2 (dba) 3 0.9g (0.785mmol, 0.5%), xylene 1500mL, sodium tert-butoxide 43.3g (314mmol), heated to reflux for 12h. After the reaction is complete, stop the reaction. Cool to room temperature, wash with water, concentrate the organic phase, and purify the obtained solid by recrystallization from toluene to obtain white powder M1.
[0049] Nitrogen protection, in the reaction flask, add 10.4g (20mmol) of intermediate M1, 34.6g (110mmol) of 4-bromophenyldiphenylamine, Pd 2 (dba) 3 0.9g (0.785mmol, 0.5%), xylene 1500mL, sodium tert-butoxide 43.3g (314mmol), heated to reflux for 12h. After the reaction is complete, stop the reaction. Cool to room temperature, wash with water, concentrate the organic phase, and purify the obtained solid ...
Synthetic example 3
[0050] Synthesis Example 3: Synthesis of Compound A39
[0051]
[0052] Nitrogen protection, in the reaction bottle, add indolo (3,2-A) carbazole 25.6g (100mmol), 2-chloro-4,6-diphenyltriazine 29.4g (110mmol), Pd 2 (dba) 3 0.9g (0.785mmol, 0.5%), xylene 1500mL, sodium tert-butoxide 43.3g (314mmol), heated to reflux for 12h. After the reaction is complete, stop the reaction. Cool to room temperature, wash with water, concentrate the organic phase, and purify the obtained solid by recrystallization from toluene to obtain white powder M1.
[0053] Nitrogen protection, in the reaction flask, add 10.4g (20mmol) of intermediate M1, 34.6g (110mmol) of 4(3-bromophenyl)-9,9-dimethylfluorene, Pd 2 (dba) 3 0.9g (0.785mmol, 0.5%), xylene 1500mL, sodium tert-butoxide 43.3g (314mmol), heated to reflux for 12h. After the reaction is complete, stop the reaction. Cool to room temperature, wash with water, concentrate the organic phase, and purify the obtained solid by recrystallizat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com