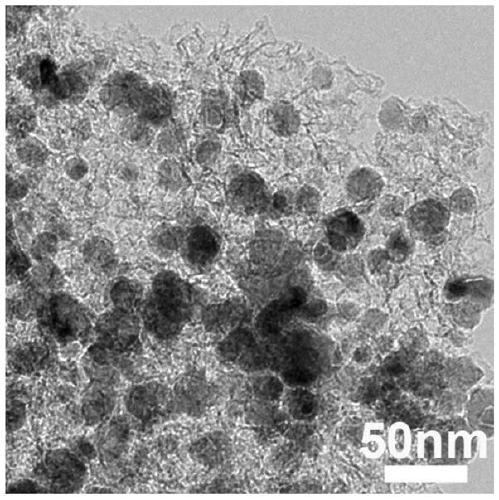
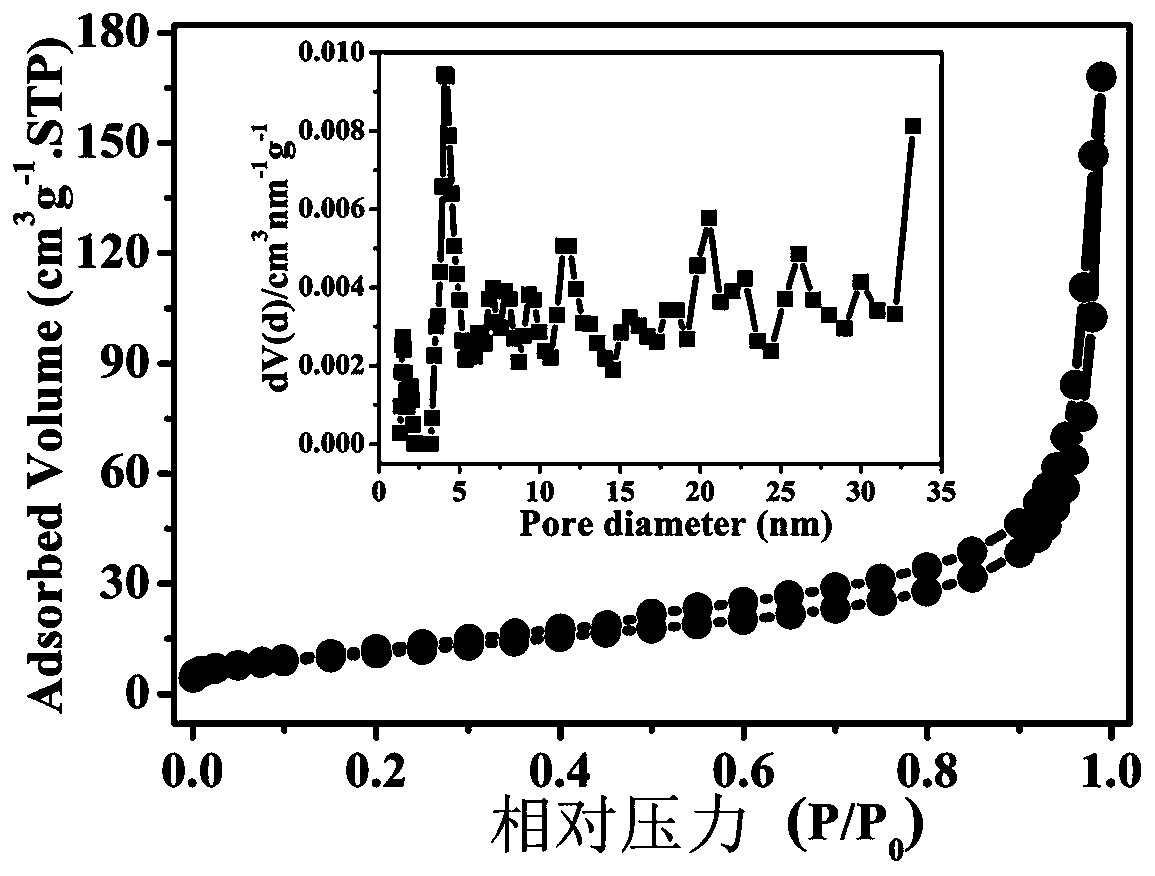
Preparation method of OER composite electrocatalyst
An electrocatalyst and uniform mixing technology, applied in the field of material chemistry, can solve the problems of weak bonding force of metal catalysts, inability of metals to be dispersed uniformly, poor stability, etc., to improve the efficiency of electrocatalytic oxygen evolution, excellent electrocatalytic performance and avoid aggregation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A preparation method for OER composite electrocatalyst, comprising the following steps:
[0037] (1) adopting TEMPO oxidation method to prepare nanocellulose suspension, the mass fraction of nanocellulose suspension is 0.3wt.%;
[0038] (2) Weigh 0.173g Ni(NO 3 ) 2 ·6H 2 O, 0.060g Fe(NO 3 ) 3 9H 2 O and 0.05g thiourea were dissolved in 10g ultrapure water, stirred for 1h;
[0039] (3) Weigh 19.1 g of nanocellulose (CNF) suspension with a weight fraction of 0.3wt.% and add it to the mixed solution in step (2), stir for 2 hours to obtain a uniform mixed solution;
[0040] (4) hydrothermally reacting the homogeneous mixed solution obtained in step (3) at 120° C. for 2 hours, and cooling naturally to obtain a hydrogel;
[0041] (5) Vacuum freeze-drying the hydrogel obtained in step (4) at -50°C for 3 days to obtain a porous airgel material;
[0042] (6) the porous airgel material obtained in the step (5) in N 2 Under protection, heat preservation at 800° C. for 2 h...
Embodiment 2
[0049] A preparation method for OER composite electrocatalyst, comprising the following steps:
[0050] (1) adopting TEMPO oxidation method to prepare nanocellulose suspension, the mass fraction of nanocellulose suspension is 0.3wt.%;
[0051] (2) Weigh 0.173g Ni(NO 3 ) 2 ·6H 2 O, 0.12g Fe(NO 3 ) 3 9H 2 O and 0.05g thiourea were dissolved in 10g ultrapure water, stirred for 1h;
[0052] (3) Weigh 19.1 g of nanocellulose (CNF) suspension with a weight fraction of 0.3wt.% and add it to the mixed solution in step (2), stir for 2 hours to obtain a uniform mixed solution;
[0053] (4) hydrothermally reacting the homogeneous mixed solution obtained in step (3) at 120° C. for 2 hours, and cooling naturally to obtain a hydrogel;
[0054] (5) Vacuum freeze-drying the hydrogel obtained in step (4) at -50°C for 3 days to obtain a porous airgel material;
[0055] (6) the porous airgel material obtained in the step (5) in N 2 Under protection, heat preservation at 800° C. for 2 ho...
Embodiment 3
[0058] A preparation method for OER composite electrocatalyst, comprising the following steps:
[0059] (1) adopting TEMPO oxidation method to prepare nanocellulose suspension, the mass fraction of nanocellulose suspension is 0.3wt.%;
[0060] (2) Weigh 0.173g Ni(NO 3 ) 2 ·6H 2 O, 0.06g Fe(NO 3 ) 3 9H 2 O and 0.05g thiourea were dissolved in 10g ultrapure water, stirred for 1h;
[0061] (3) Weighing 51.1 g of nanocellulose (CNF) suspension with a weight fraction of 0.3wt.% and adding it to the mixed solution in step (2), stirring for 2 hours to obtain a uniform mixed solution;
[0062] (4) hydrothermally reacting the homogeneous mixed solution obtained in step (3) at 120° C. for 2 hours, and cooling naturally to obtain a hydrogel;
[0063] (5) Vacuum freeze-drying the hydrogel obtained in step (4) at -50°C for 3 days to obtain a porous airgel material;
[0064] (6) the porous airgel material obtained in the step (5) in N 2 Under protection, heat preservation at 800° C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com