Dipyridine iron complex, and preparation method and applications thereof
A bipyridine iron and complex technology, applied in the field of isoprene catalyzed polymerization, can solve the problems of poor microstructure controllable adjustment ability and high catalyst cost, and achieve the effects of excellent selectivity, high molecular weight and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
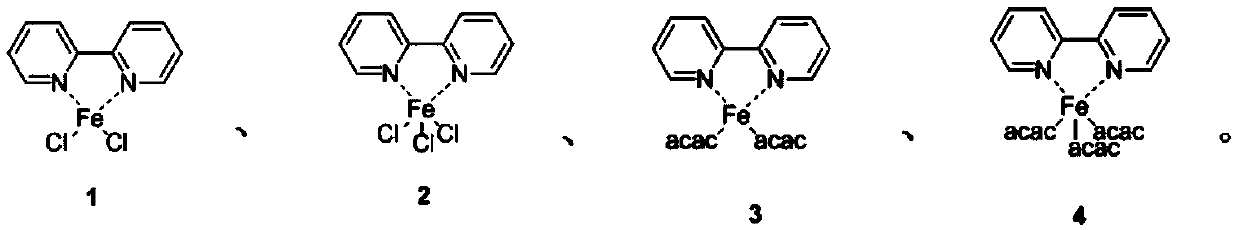
[0028] Example 1. Synthesis of bipyridyl iron complex 1.
[0029] The bipyridyl iron complex described in the present embodiment has the structural formula:
[0030] Prepared by the following method:
[0031] Add anhydrous FeCl to a 50 mL Schlenk bottle under argon atmosphere 2 (164.8mg, 1.3mmol), dissolved in 10mL of absolute ethanol at 60°C; then a solution of 2,2'-bipyridine (203.0mg, 1.3mmol) in ethanol (10mL) was added dropwise to the system. React at 60°C for 1 hour. The orange-red complex precipitated from the system, was filtered, washed twice with cold ethanol, concentrated to remove the solvent, and dried in vacuum for 12 hours to obtain orange-red solid product 1 with a yield of 74%.
[0032] Mass Spectrometry: C 10 h 8 Cl 2 FeN 2 :[M-Cl]+: theoretical value: 246.9720; measured value: 246.9718.
[0033] Elemental Analysis: C 10 h 8 Cl 2 FeN 2 : Theoretical value: C, 42.45%; H, 2.85%; N, 9.90%; Measured value: C, 42.72%; H, 2.67%; N, 9.82%.
Embodiment 2
[0034] Embodiment 2: the synthesis of bipyridyl iron complex 2.
[0035] The bipyridyl iron complex described in the present embodiment has the structural formula:
[0036] Prepared by the following method:
[0037] Add anhydrous FeCl to a 50 mL Schlenk bottle under argon atmosphere 3 (210.6mg, 1.3mmol), dissolved in 10mL of absolute ethanol at 60°C; then a solution of 2,2'-bipyridine (203.0mg, 1.3mmol) in ethanol (10mL) was added dropwise to the system. React at 60°C for 1 hour. The yellow complex precipitated from the system, filtered, washed twice with cold ethanol, concentrated to remove the solvent, and dried in vacuo for 12 hours to obtain apricot yellow solid product 2 with a yield of 77%.
[0038] Mass Spectrometry: C10 h 8 Cl 3 FeN 2 : [M-Cl]+: theoretical value: 281.9408; measured value: 281.9404.
[0039] Elemental Analysis: C 10 h 8 Cl 3 FeN 2 : Theoretical value: C, 37.73%; H, 2.53%; N, 8.80%; Measured value: C, 37.98%; H, 2.26%; N, 8.55%.
Embodiment 3
[0040] Embodiment 3: the synthesis of bipyridyl iron complex 3.
[0041] The bipyridyl iron complex described in the present embodiment has the structural formula:
[0042] Prepared by the following method:
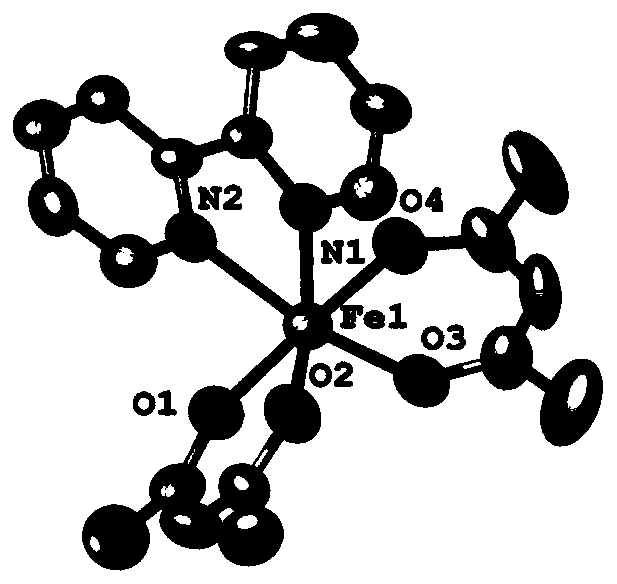
[0043] Add anhydrous Fe(acac) to a 50 mL Schlenk bottle under argon atmosphere 2 (127.0mg, 0.5mmol), dissolved in 6mL of absolute ethanol at 60°C; then a solution of 2,2'-bipyridine (78.1mg, 0.5mmol) in ethanol (4mL) was added dropwise to the system. React at 60°C for half an hour, then return to room temperature and stir overnight. The filtrate was collected by filtration, concentrated, washed twice with cold ethanol, and dried in vacuum for 12 h to obtain product 3 as a brownish yellow solid with a yield of 68%.
[0044] Mass Spectrometry: C 20 h 22 FeN 2 o 4 :[M+H]+: theoretical value: 411.1002; measured value: 410.0998.
[0045] Elemental Analysis: C 20 h 22 FeN 2 o 4 : Theoretical value: C, 58.55%; H, 5.41%; N, 6.83%; Measured value: C, 58.34%; H, 5.53%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com