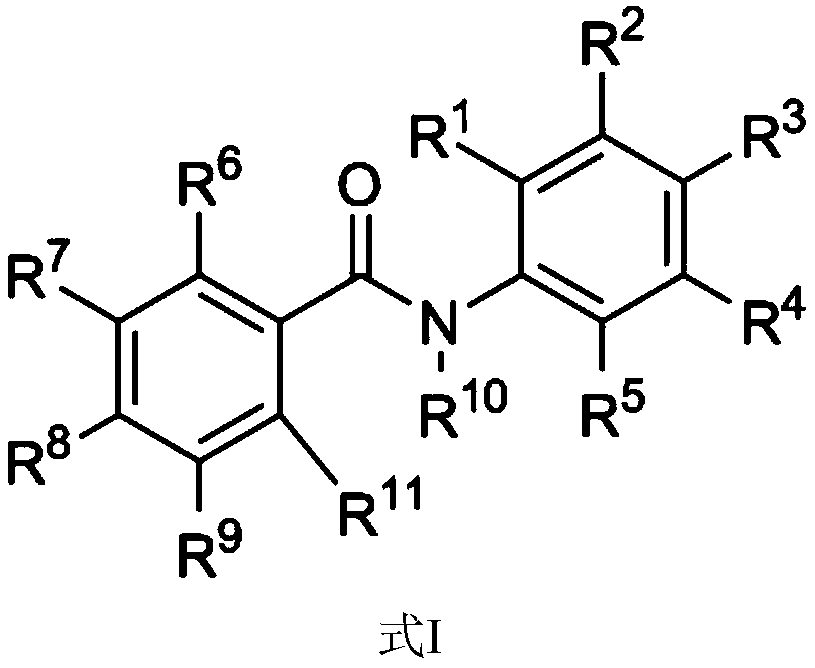
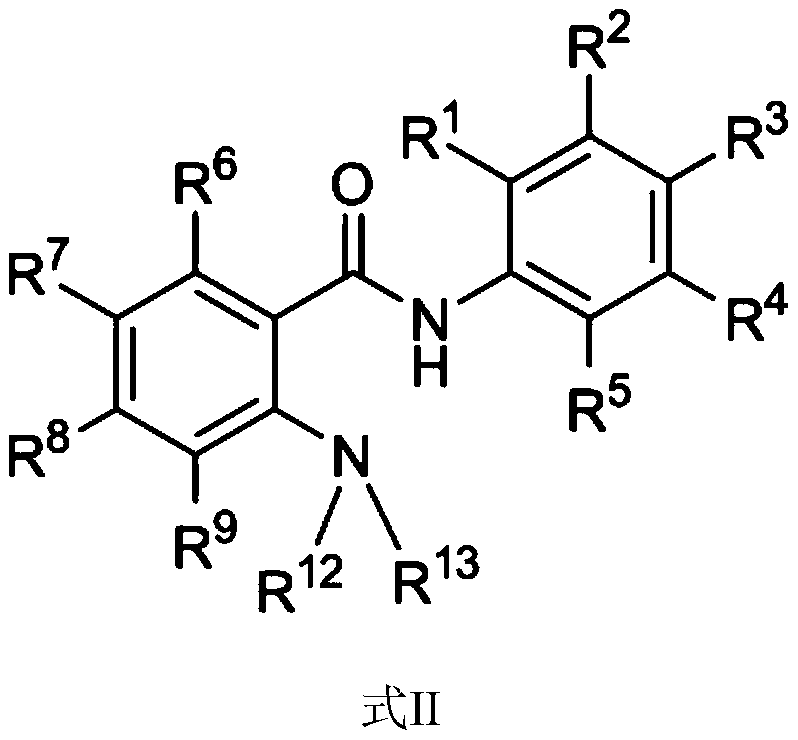
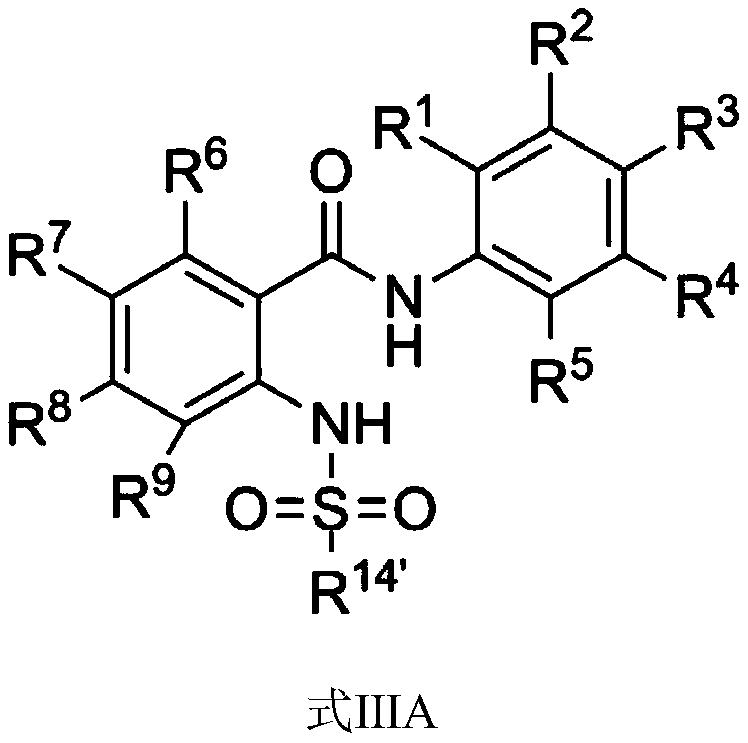
Amide compound and application thereof in treating cancer
A compound and solvate technology, applied in the field of medicine, can solve problems such as obvious side effects and no optimal treatment plan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] Example 1: 5-chloro-N-(2-chloro-4-trifluoromethylphenyl)-2-aminobenzamide (1)
[0145]
[0146] The first step: 5-chloro-N-(2-chloro-4-trifluoromethylphenyl)-2-nitrobenzamide (1-1)
[0147] Under nitrogen protection, 5-chloro-2-nitrobenzoic acid (5.0g, 24.8mmol), triethylamine (5.0g, 24.8mmol) and 3-chloro-4-aminotrifluorotoluene (4.6g, 23.6mmol ) was added to 50mL xylene, heated to 110°C, and phosphorus trichloride (3.4g, 24.8mmol) was slowly added dropwise. At 110°C, stir for 2h. Cool to room temperature and spin dry under vacuum. Add 100mL of water, filter the solid with suction, wash the filter cake with water, beat with a small amount of ethyl acetate, filter the solid with suction, and dry to obtain a white solid 5-chloro-N-(2-chloro-4-(trifluoromethyl) Phenyl)-2-nitrobenzamide (8.2 g, 21.6 mmol). Yield: 87.2%. MS(ESI)m / e396.0(M+18) + . 1 H NMR (400MHz, CDCl 3 )δ8.63 (d, J=8.5Hz, 1H), 8.16 (d, J=8.4Hz, 1H), 7.99 (s, 1H), 7.71 (s, 1H), 7.69-7.57 (m, 3H)....
Embodiment 2
[0150] Example 2: 5-Chloro-N-(2-chloro-4-(trifluoromethyl)phenyl)-2-(methylsulfonamido)benzamide (2)
[0151] 2-Amino-5-chloro-N-(2-chloro-4-(trifluoromethyl)phenyl)benzamide (100.0mg, 0.28mmol) and 3mL pyridine were slowly added dropwise with methanesulfonyl chloride (65.6 mg, 0.57mmol). Heat to 40°C and stir overnight. Dichloromethane and water were added for extraction, and the organic layer was washed with 1N dilute hydrochloric acid, then with saturated brine, dried, spin-dried, and purified by silica gel column chromatography. 5-Chloro-N-(2-chloro-4-(trifluoromethyl)phenyl)-2-(methylsulfonamido)benzamide (60.0 mg, 0.14 mmol) was obtained as a white solid. Yield: 49.0%. MS(ESI)m / e427.0(M+H) + .
Embodiment 3
[0152] Example 3: 5-chloro-N-(2-chloro-4-trifluoromethylphenyl)-2-propionamidobenzamide (3)
[0153] In a 25ml round bottom flask, put 5-chloro-N-(2-chloro-4-trifluoromethylphenyl)-2-aminobenzamide (0.2mmol, 70mg), propionyl chloride (1.2eq, 23mg) , dichloromethane 1ml and TEA (2eq, 40mg), stirred at room temperature overnight, the reaction solution was washed with dilute hydrochloric acid, washed with saturated brine, dried, spin-dried, slurried with ice dichloromethane, filtered to obtain 10mg of white solid, yield 13%
[0154] MS m / z (ESI): 210.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com