Hybrid peptide having functions of detoxification, anti-inflammatory, anti-apoptosis, protection of intestinal barrier and promotion of wound healing and application thereof
A technology of hybrid peptides and polypeptides, which is applied in the field of genetic engineering and biological preparations, can solve problems such as unsuitable for continuous use, achieve strong anti-inflammatory activity, inhibit cell apoptosis and intestinal barrier damage, and reduce expression levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Design and acquisition of embodiment 1 hybrid peptide C-L
[0044] By studying the sequence, structure and the relationship between sequence structure and function of polypeptide LL-37 and cecropin A, the protein molecular design technology was used to carry out polypeptide Cecropin A (its amino acid sequence is shown in SEQ ID NO.2) and LL- 37 (whose amino acid sequence is shown in SEQ ID NO.3) to obtain hybrid peptide C-L, whose amino acid sequence is shown in SEQ ID NO.1.
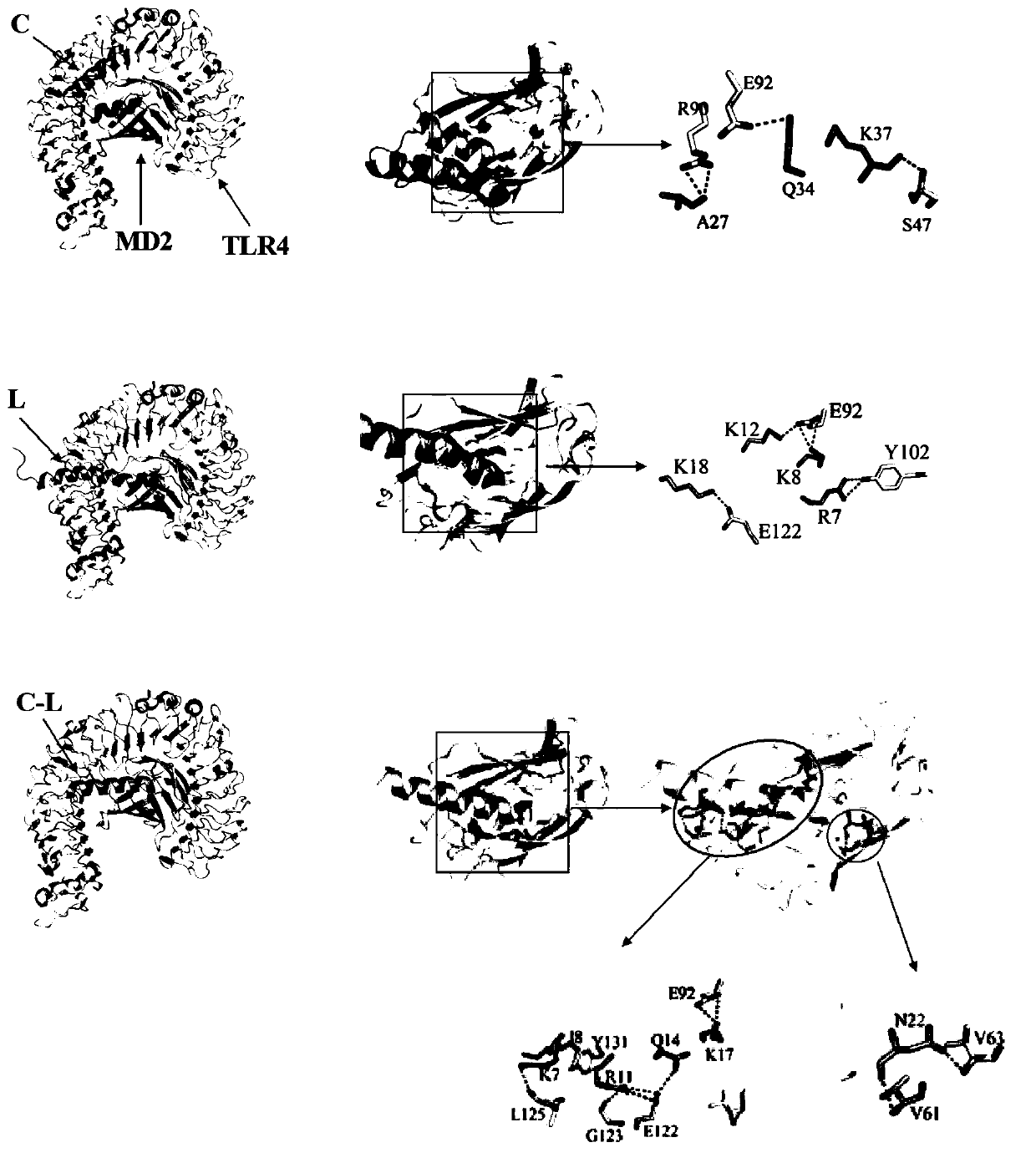
[0045] Myeloid differentiation protein-2 (MD-2) binds to TLR4 (Toll receptor 4) to form a TLR4 / MD2 complex receptor. LPS mainly binds to TLR4 / MD2 on the surface of the cell membrane to activate downstream inflammatory signaling pathways such as NF-κB and MAPK, leading to inflammation outbreaks. Therefore, TLR4 / MD2 is a regulatory molecule in innate immunity. It has a wide range of biological functions in pathophysiological processes.
[0046] The present invention studies the molecular dynamics ...
Embodiment 2
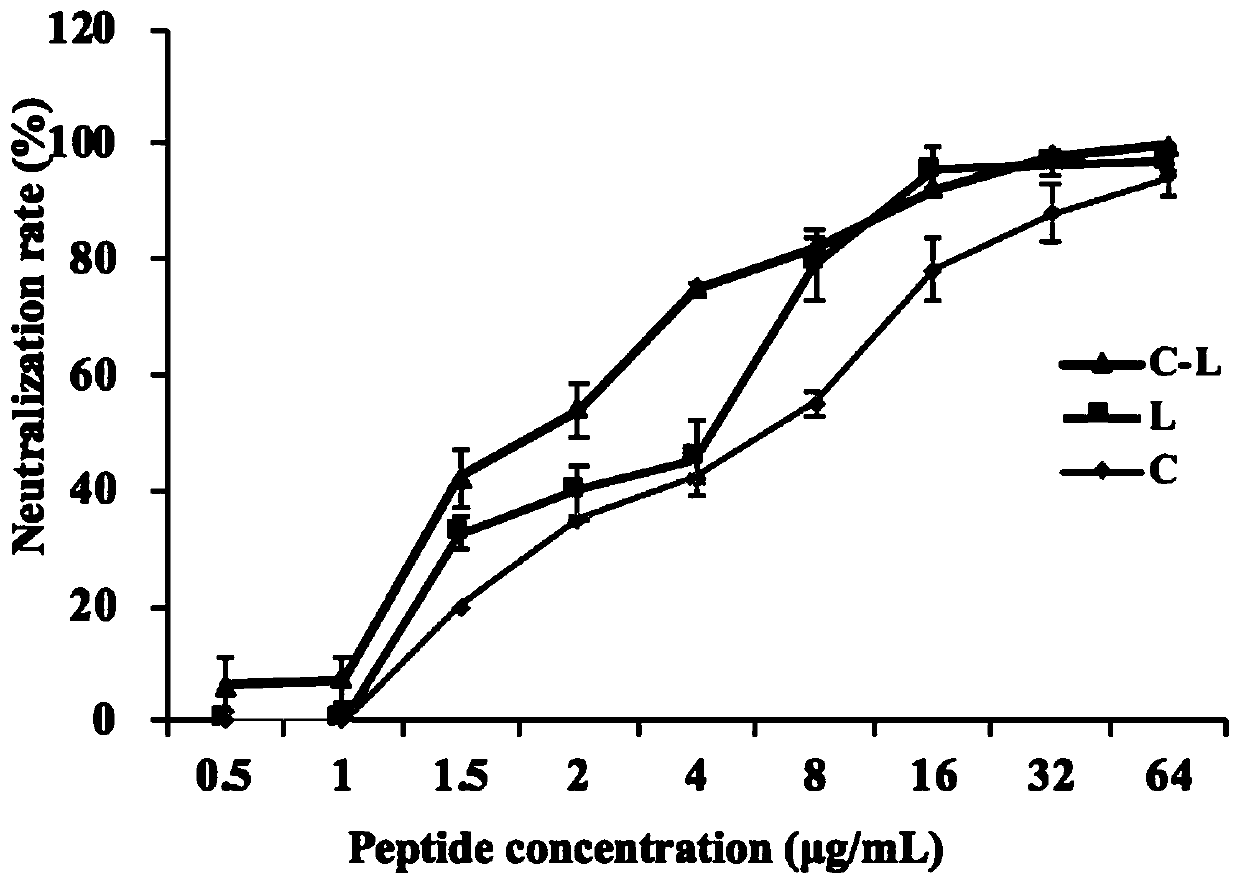
[0053] Example 2 The neutralization and digestion effect of hybrid peptide C-L on LPS
[0054] Dissolve and dilute the hybrid peptide C-L and its parent peptide LL-37 and Cecropin A into solutions of different concentrations (0-64 μg / mL) using pyrogen-free endotoxin test water, and take 100 μL of the above-mentioned peptide solutions of each concentration and LPS (1EU / mL) mixed. After incubation at 37°C for 30 min, the neutralization rate of peptides Cecropin A, LL-37, and C-L to LPS was detected using a chromogenic substrate Limulus kit. The results are shown in Figure 2. Peptide C-L has a higher neutralization and digestion activity of LPS, and its neutralization activity is higher than that of its parent peptide LL-37 and Cecropin A. When 50% LPS neutralization rate was reached, the concentrations of C-L, L, and C were close to 2, 4, and 8 μg / mL, respectively, indicating that the ability of C-L to neutralize LPS was about 2 times and 4 times that of its parent peptides L a...
Embodiment 3
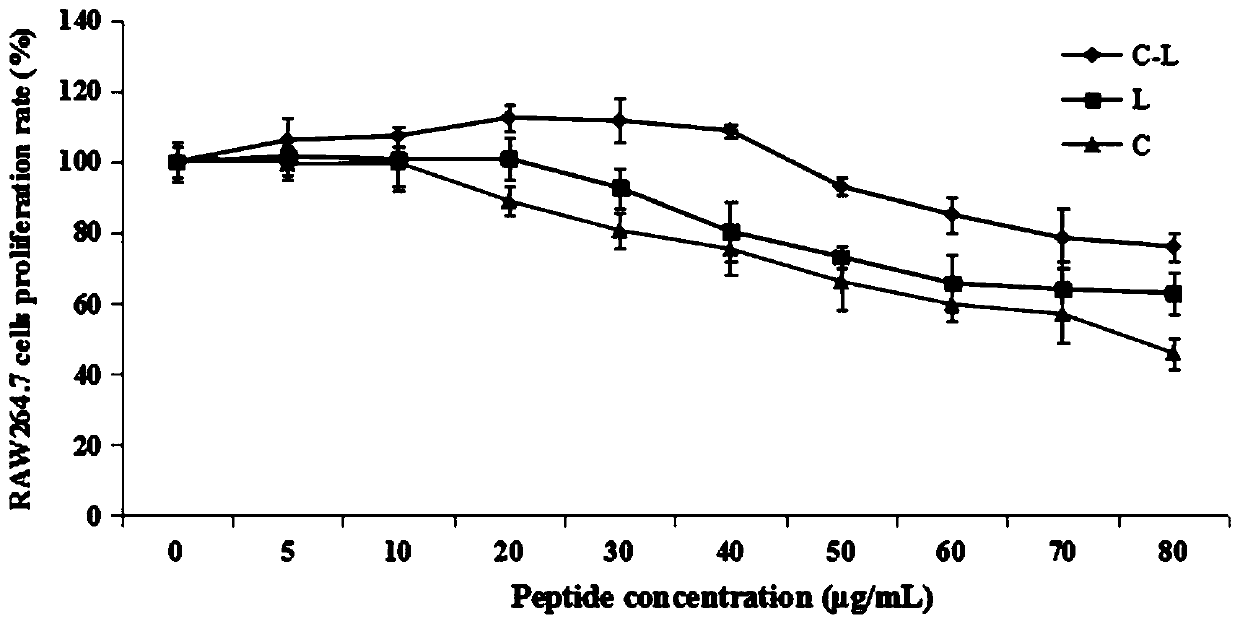
[0055] Example 3 Effect of Hybrid Peptide C-L on Survival Rate of Mouse Macrophage Cells
[0056] The macrophage RAW264.7 in the logarithmic growth phase was inoculated in a 96-well plate, and the initial cell culture density was 1×10 4 cells / mL, 100 μL per well, at 37°C, 5% CO 2 After culturing overnight under certain conditions, a series of concentrations of C-L, LL-37 and Cecropin A (0-100 μg / mL) solutions were added, and after culturing for 24 hours, the CCK8 method was used to detect the effect of polypeptide C-L on the survival rate of mouse macrophages. influences. The result is as image 3 As shown, the cytotoxicity of hybrid peptide C-L was significantly lower than that of its parent peptide, and the survival rate of macrophages was greater than 80% in the concentration range of 0-70 μg / mL, indicating that C-L had lower cytotoxicity and higher security.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com