A kind of indole compound and its synthesis method and application
A synthesis method and compound technology, applied in the field of indole compounds and their synthesis, can solve problems such as the inability to overcome the drug resistance of tumor cells, and achieve the effects of small side effects, good inhibitory effect, and good resistance to drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The present invention also provides a kind of synthetic method of indole compound, comprising:
[0048]The compound of formula (II) structure, palladium acetate, benzoquinone compound and organic acid are dissolved in solvent and reacted to obtain the compound of formula (I) structure;
[0049]
[0050] where the R 1-2 , R 1-4 , R 1-6 independently selected from hydrogen, C1-C6 alkyl, C1-C6 alkoxy;
[0051] The R 1-3 , R 1-5 independently selected from hydrogen or halogen;
[0052] and the R 1-2 , R 1-3 , R 1-4 , R 1-5 , R 1-6 not simultaneously hydrogen;
[0053] The R 2-0 selected from hydrogen, C1-C5 methyl or C1-C5 methoxy;
[0054] The R 2-2 selected from hydrogen, C1-C6 methyl or C1-C6 methoxy;
[0055] The R 2-3 selected from hydrogen, C1-C6 methyl or C1-C6 methoxy;
[0056] The R 2-4 selected from hydrogen, C1-C6 methyl or C1-C6 methoxy;
[0057] The R 2-5 selected from hydrogen, C1-C6 methoxy or halogen;
[0058] The R 2-6 selected from...
preparation example
[0076] 1) The preparation of intermediate allylaniline compounds, taking N-allylaniline as an example, is shown in the following formula:
[0077]
[0078] Aniline (1mmol) and K 2 CO 3 (1.5mmol) was added into anhydrous DMF, then allyl bromide (1mmol) was added dropwise, stirred for 12h, quenched with water, extracted with ethyl acetate, washed with saturated brine, anhydrous Na 2 SO 4 Drying and column separation (petroleum ether) gave N-allylaniline with a yield of 60% and a purity of 99.99%.
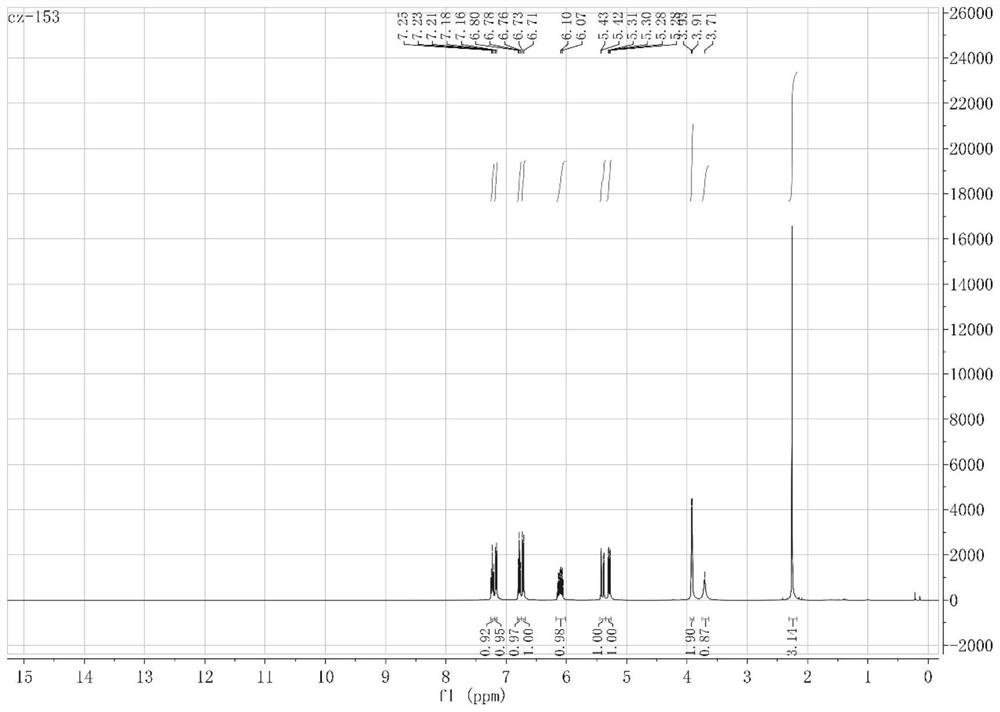
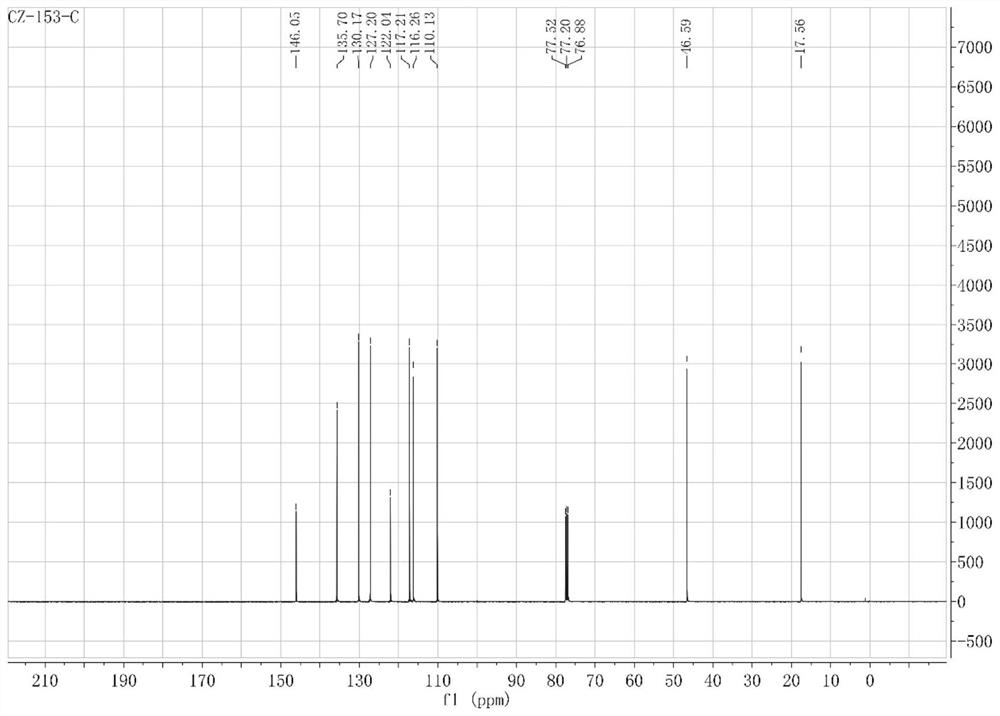
[0079] The obtained compound is identified, and its NMR results are as follows: 1 H NMR (500MHz, CDCl 3 )δ7.25–7.20(m,2H),6.93(t,J=7.4Hz,1H),6.80(d,J=7.9Hz,1H),6.12(dq,J=11.6,6.2Hz,1H), 5.29(t, J=13.2Hz, 2H), 3.78(s, 2H), 3.45(d, J=6.2Hz, 2H). 13 C NMR (126MHz, CDCl 3 )δ143.76(s), 134.89(s), 129.08(s), 126.46(s), 122.91(s), 117.76(s), 115.01(s), 114.74(s), 35.37(s).
[0080] 2) The preparation of intermediate 2-allyl aniline compounds, taking 2-allyl aniline as an example, ...
Embodiment
[0093] According to the preparation method provided in the preparation example, different raw materials are selected to obtain a series of R 1 and R 2 Indole compounds with different substituents are denoted as 5b~5u. The specific reaction process and the obtained products are shown in the following reaction process;
[0094]
[0095] The product detection that obtains to each step reaction, wherein, figure 2 is the hydrogen spectrum of allylaniline compound 2b, figure 2 It is the carbon spectrum of allylaniline compound 2b; image 3 is the hydrogen spectrum of allylaniline compound 3b, Figure 4 It is the carbon spectrum of allylaniline compound 3b; Figure 5 Be the hydrogen spectrum of N-(allylphenyl) benzamide compound 4k; Figure 6 Be the carbon spectrum of N-(allylphenyl) benzamide compound 4k; Figure 7 It is the hydrogen spectrum of indole compound 5k, Figure 8 It is the carbon spectrum of the indole compound 5k; specifically, the NMR data of the compound o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com