A pt@pcn-224 photocatalyst for preparing artemisinin from dihydroartemisinic acid and its preparation method
A technology of dihydroartemisinic acid and photocatalyst, applied in organic compound/hydride/coordination complex catalyst, chemical instrument and method, physical/chemical process catalyst, etc., can solve the problem of high cost, high yield and conversion rate low problems, to achieve the effect of high porosity, lower production costs, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
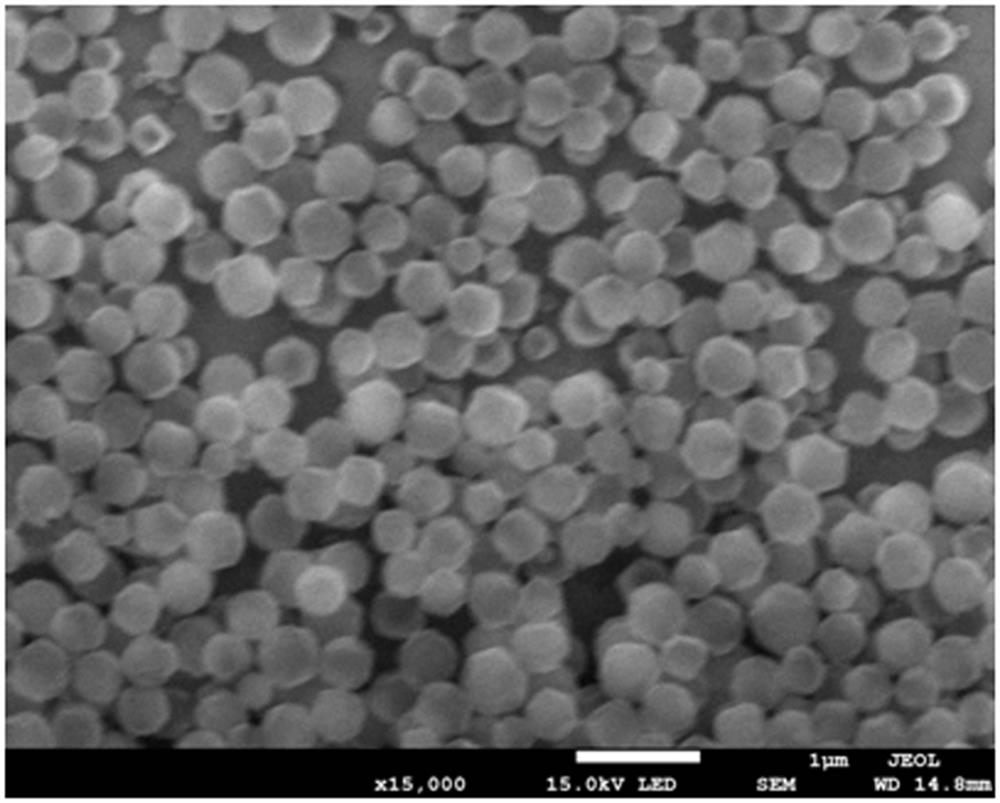
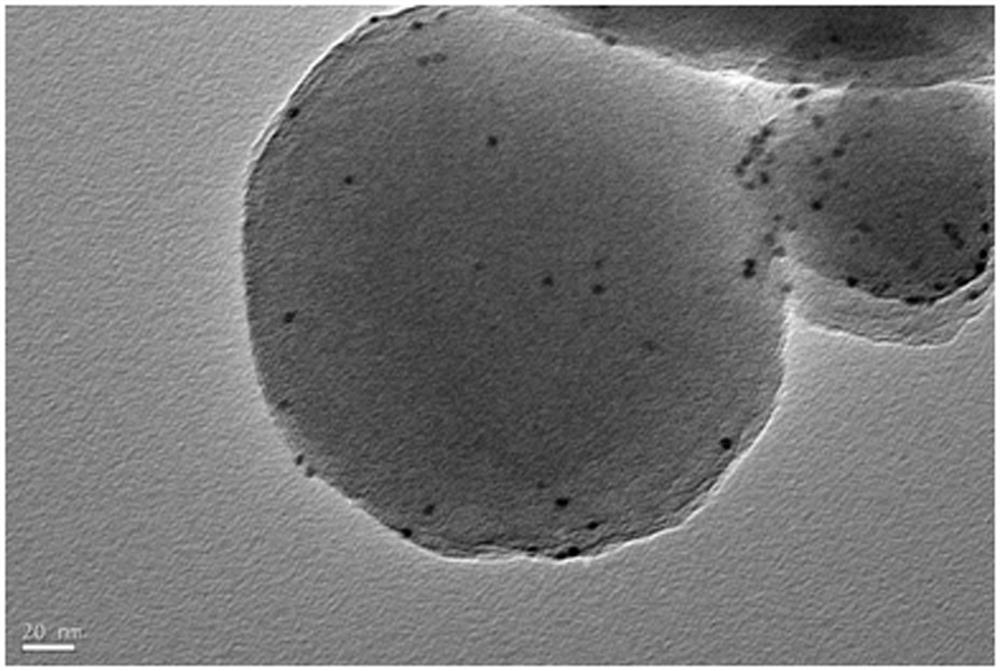
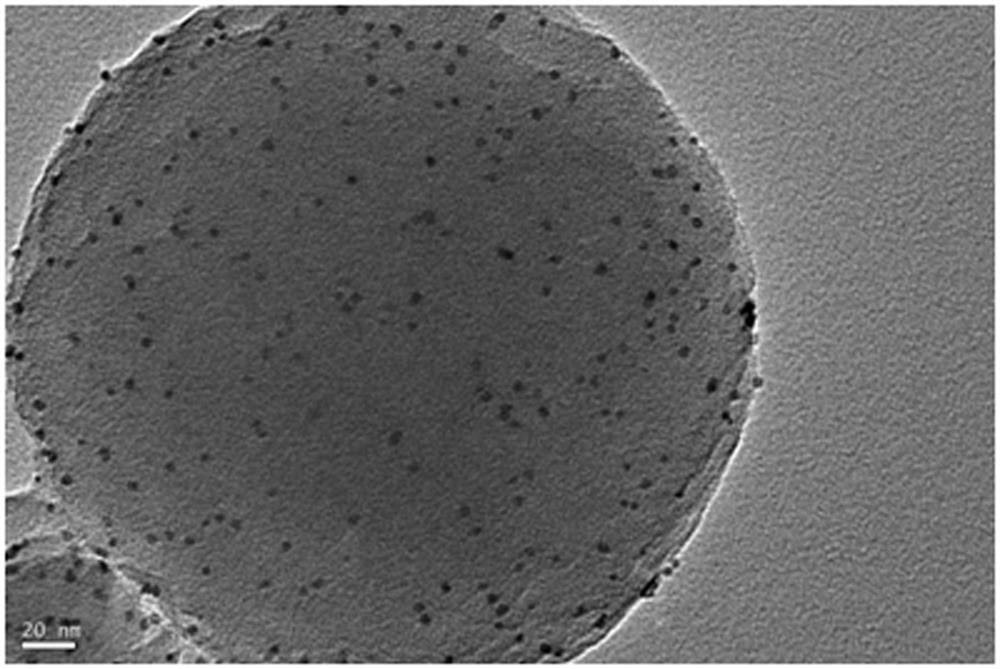
[0033] 120mg Zr 6 Metal clusters are dissolved in 5ml of mixed solution A of N,N-dimethylacetamide and acetic acid, and the volume ratio of N,N-dimethylacetamide and acetic acid in solution A is any one of 0:5 to 4:1. 60 mg of phenylporphyrin tetracarboxylate was dissolved in 2.5 ml of N,N-dimethylacetamide to obtain mixed solution B. Pt nanoparticles (1 mg, 3 mg, 5 mg) were dissolved in N, N-dimethylacetamide with a volume of 2.5 ml to obtain a mixed solution C. The three are completely mixed under the stirring condition that the rotating speed of the magnet is 900 rpm, stirring is carried out at 25 degrees Celsius, and the stirring time is controlled between 0.5-24 hours. The preliminary products were collected by centrifugation, washed three times with N, N-dimethylacetamide, washed once with acetone, and dried to obtain pure phases of Pt@PCN-224-1, Pt@PCN-224-3, and Pt@PCN-224-5, respectively. for the final product.
Embodiment 2
[0035] 120mg Zr 6 Metal clusters were dissolved in 5ml of mixed solution A of N,N-dimethylacetamide and acetic acid, and the volume ratio of N,N-dimethylacetamide and acetic acid in solution A was 2:3. Dissolve 60mg of TCPP-Zn (or TCPP-Co, TCPP-Ni) in 2.5ml of N, N-dimethylacetamide to obtain mixed solution B. 1 mg of Pt nanoparticles was dissolved in 2.5 ml of N,N-dimethylacetamide to obtain a mixed solution C. The three are completely mixed under the stirring condition that the rotating speed of the magnet is 900 rpm, and the stirring is carried out at 25 degrees Celsius for 9 hours. The preliminary products were collected by centrifugation, washed three times with N, N-dimethylacetamide, washed once with acetone, and dried to obtain pure phases of Pt@PCN-224-Zn, Pt@PCN-224-Co, and Pt@PCN-224-Ni respectively. for the final product.
Embodiment 3
[0037] Dihydroartemisinic acid (25 mg, 0.106 mmol), Pt@PCN-224 obtained in Example 1 and Example 2 or its derivatives (0.002 mmol, based on porphyrin), trifluoroacetic acid (8 μL), dispersed in 5 ml di A mixed solution was obtained in methyl chloride. The mixed solution was slowly bubbled with oxygen for 3 hours under LED lighting conditions. Remove the dichloromethane contained in the reactant, by1 H NMR further proved the conversion rate of dihydroartemisinic acid. Finally, the conversion rate of Pt@PCN-224-1 was 98%, and the yield rate was 45%. The conversion of Pt@PCN-224-3 was 99%, and the yield was 50%. The standardization rate of Pt@PCN-224-5 was 99%, and the yield rate was 51%. The conversion rate of Pt@PCN-224-Zn is 99%, the yield rate is 54%, the conversion rate of Pt@PCN-224-Co is 99%, the yield rate is 53%, and the conversion rate of Pt@PCN-224-Ni is 99%. , and the yield was 52%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com