P-phenyl substituted alpha-diimine palladium catalyst and preparation method thereof
A phenyl-substituted, diimide palladium technology, applied in the field of lubricating oil, can solve the problems of harsh oligomerization reaction conditions and difficult process, and achieve the effects of high viscosity, improved catalytic activity and high viscosity index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
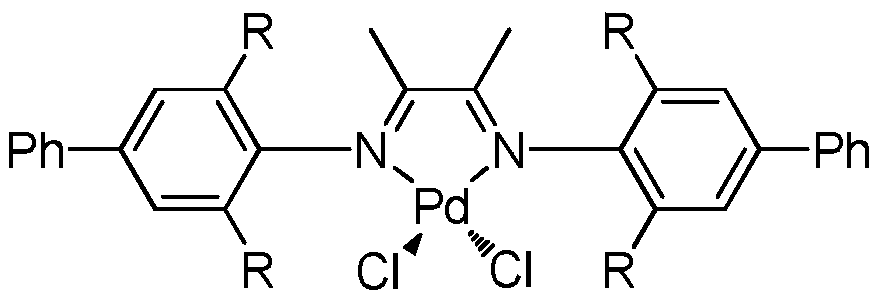
[0032] This embodiment is to prepare the α-diimine palladium catalyst substituted by p-phenyl, which is denoted as catalyst C1 here, and its specific preparation process is as follows:
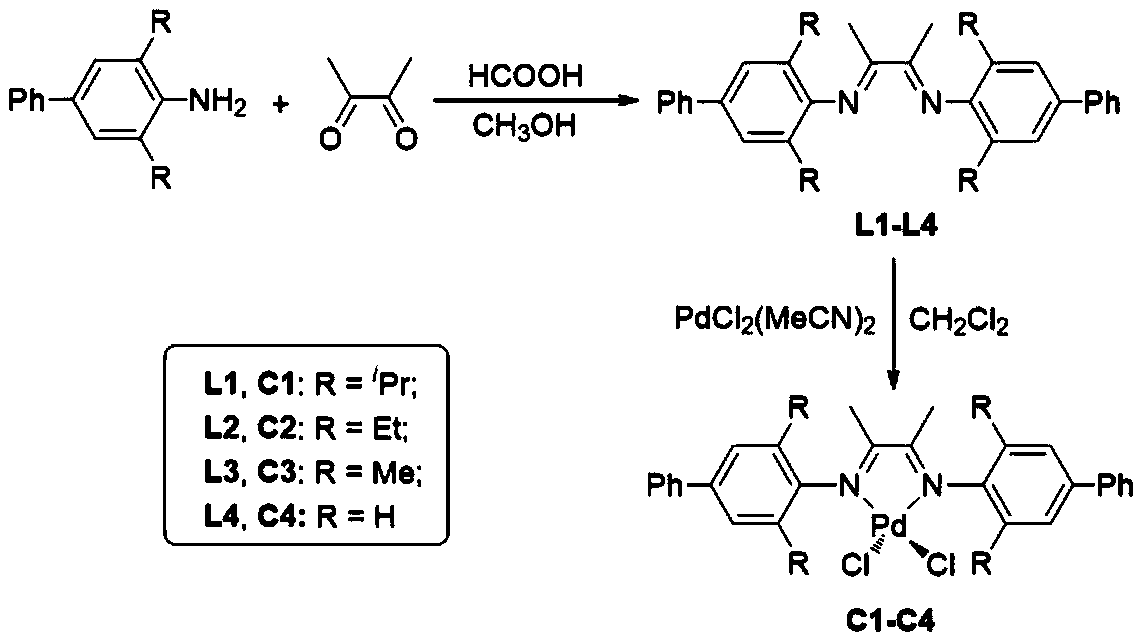
[0033] S1: Synthesis of Ligand L1:
[0034] 4-Phenyl-2,6-diisopropylaniline (5.07 g, 20 mmol) and 2,3-butanedione (0.85 g, 10 mmol) were dissolved in 30 mL of anhydrous methanol, and 0.25 g of formic acid was added under stirring , reflux at 45°C for 40h, remove the solvent to obtain a crude product, and then use CH 3 OH / CH 2 Cl 2 (v / v=15:1) mixed solvent recrystallization, precipitated solid precipitate, filtered and dried to obtain ligand 4.41g, yield 74%.
[0035] Its reaction formula is as follows:
[0036]
[0037] S2: Synthesis of Catalyst C1:
[0038] in N 2 Under protection, the above ligand (2.78 g, 0.50 mmol) was added to a 100 mL dry flask, followed by PdCl 2 (MeCN) 2 (1.3g, 5mmol) and 30mL CH 2 Cl 2 , stirred at room temperature for 24 h, filtered the suspension, filte...
Embodiment 2
[0042] This embodiment is to prepare the α-diimine palladium catalyst substituted by p-phenyl group, which is denoted as catalyst C2 here, and its specific preparation process is as follows:
[0043] S1: Synthesis of Ligand L2:
[0044] 4-Phenyl-2,6-diethylaniline (4.51g, 20mmol) and 2,3-butanedione (0.90g, 10mmol) were dissolved in 32mL of anhydrous methanol, and 0.27g of formic acid was added under stirring, Reflux the reaction at 20°C for 12h, remove the solvent to obtain a crude product, and then use CH 3 OH / CH 2 Cl 2 (v / v=15:1) mixed solvent recrystallization, precipitated solid precipitate, filtered and dried to obtain ligand 4.90g, yield 75%.
[0045] S2: Synthesis of Catalyst C2:
[0046] in N 2 Under protection, the above ligand (2.80 g, 0.50 mmol) was added to a 100 mL dry flask, followed by PdCl 2 (MeCN) 2 (1.3g, 5mmol) and 30mL CH 2 Cl 2 , stirred at room temperature for 12 h, filtered the suspension, filtered the mixture, and removed the solvent in vacuo....
Embodiment 3
[0050] This embodiment is to prepare the α-diimine palladium catalyst substituted by p-phenyl group, which is denoted as catalyst C3 here, and its specific preparation process is as follows:
[0051] S1: Synthesis of Ligand L3:
[0052] Dissolve 4-benzhydryl-2,6-dimethylaniline (3.95 g, 20 mmol) and 2,3-butanedione (0.83 g, 10 mmol) in 29 mL of anhydrous methanol, and add 0.24 g of Formic acid, reflux at 55°C for 60h, remove the solvent to obtain a crude product, and then use CH 3 OH / CH 2 Cl 2 (v / v=15:1) mixed solvent recrystallization, precipitated solid precipitate, filtered and dried to obtain ligand 4.71g, yield 76%.
[0053] S2: Synthesis of Catalyst C3:
[0054] in N 2 Under protection, the above ligand (2.82 g, 0.50 mmol) was added to a 100 mL dry flask, followed by PdCl 2 (MeCN) 2 (1.3g, 5mmol) and 30mL CH 2 Cl 2 , stirred at room temperature for 48 h, filtered the suspension, filtered the mixture, and removed the solvent in vacuo. The obtained solid was washe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com