Preparation method of candesartan cilexetil tablets
A technology for candesartan medoxomil and sartan medoxomil tablets, which are applied in directions such as medical preparations without active ingredients, medical preparations containing active ingredients, and pill delivery, etc., can solve the problem of decreased drug stability and unfavorable commercial production. , affecting the appearance of the product, etc., to achieve a good dissolution effect, avoid the decline of drug stability, and avoid the effect of reducing the dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A preparation method of candesartan cilexetil tablet, comprising the following steps;
[0032] (1) Weighing and preparing materials: weighing and preparing materials according to the prescription ratio in the table below;
[0033]
[0034] (2) Preparation method;
[0035] The candesartan cilexetil, lactose, cornstarch, hydroxypropyl cellulose, and carmellose calcium that were taken by weighing the prescription amount were passed through a 50-mesh sieve three times using a swinging granulator, and were used for subsequent use; the polyethylene glycol 6000 dissolved in the prescription amount In 95% ethanol, stir and control the temperature in a water bath of 50-60°C, heat and dissolve to prepare a wetting agent for later use; place the sieved and mixed raw and auxiliary materials in a granulator for dry mixing for 5 minutes, and set the stirring speed of the granulator to 160rpm, the shear rate is 1500rpm. Then add a 95% ethanol solution of polyethylene glycol 6000 ...
Embodiment 2
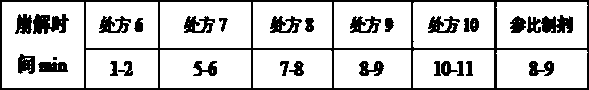
[0037] The candesartan cilexetil tablets prepared by different prescriptions in Example 1 were placed at 60°C for 10 days, and a high temperature experiment was carried out to investigate the related substances of the product. The experimental results are as follows:
[0038]
[0039] According to the measurement results, it can be known that the product produced by the preparation method of the present invention, when the ratio of the dosage of candesartan cilexetil and polyethylene glycol 6000 in the prescription is 2:1, the related substances in the product are relatively higher than the related substances in other prescriptions. is the most stable.
Embodiment 3
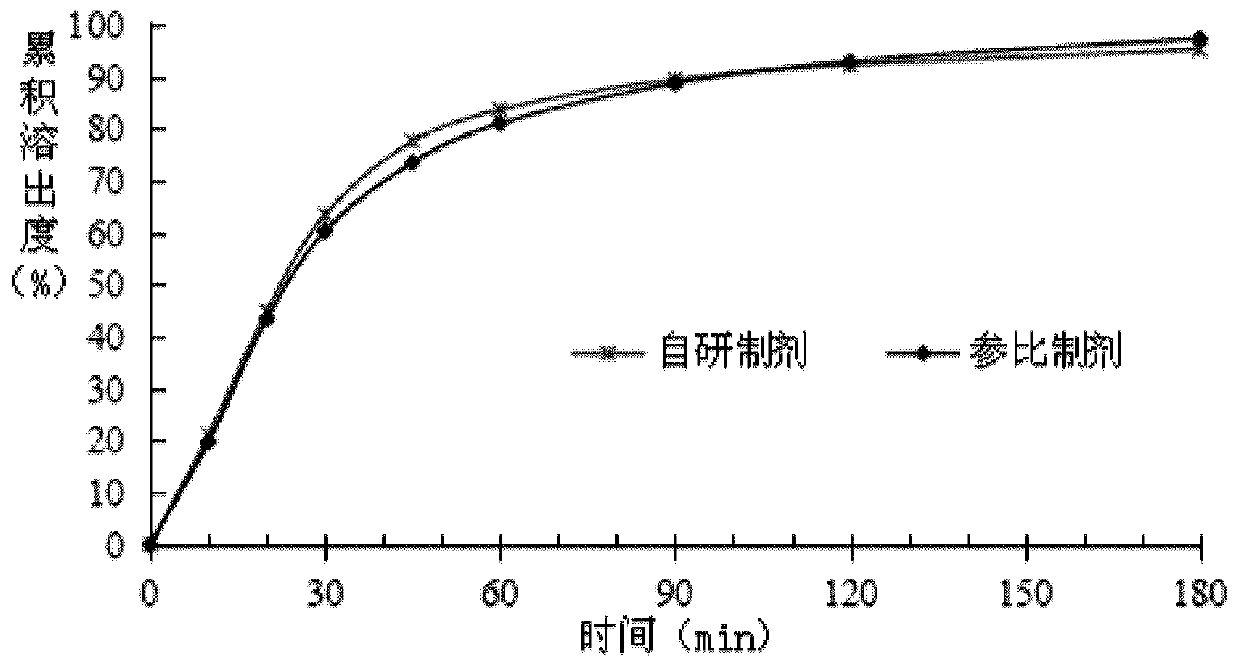
[0041]Press 2015 version " Chinese Pharmacopoeia " four general rules 0931 dissolution rate and release rate determination method second method, measure the dissolution rate of different prescription products in embodiment 1, wherein dissolution medium: 1% polysorbate 20 aqueous solution 900ml, rotating speed: 50rpm, The measurement results are as follows;
[0042]
[0043] In the prior art, on the one hand, the addition of polyethylene glycol 6000 can improve the stability of the medicinal ingredients in the solid preparation, but on the other hand, it will also lead to a decrease in the disintegration of the solid preparation, so that the drug can be released from the solid preparation. Dissolution properties were significantly reduced. According to the above measurement results, it can be known that the addition of polyethylene glycol 6000 has little effect on the dissolution rate of the product by adopting the preparation method of the present invention, and does not ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com