Chromon-substituted 2-hydroxypyrrole derivatives, and synthesis method and application thereof
A technology of hydroxypyrrole and synthetic method, which is applied in the direction of drug combination, organic chemical method, antipyretic, etc., can solve the problems of difficult requirements and harsh conditions, and achieve good anti-inflammatory activity, low synthetic cost, and easy-to-obtain synthetic raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
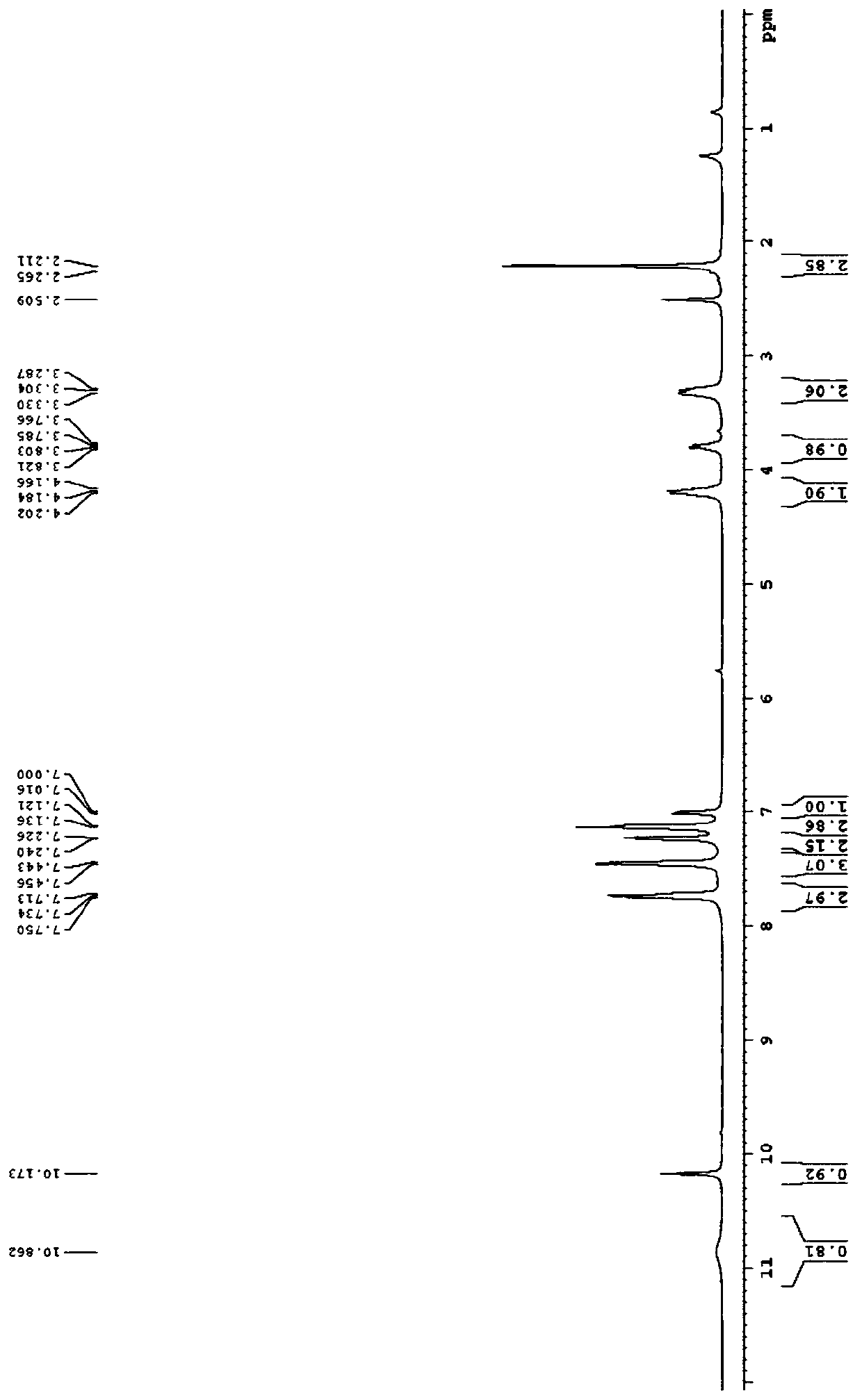
[0043] Example 1: The structural formula of the 2-hydroxypyrrole derivative substituted by the chromone group in this example is shown in the following formula
[0044]
[0045] Named (E)-3-(7-benzoyl-5-hydroxy-5-(p-tolyl)-2,3-dihydro-1H-pyrrolo[1,2-a]imidazole-6-( 5H)-subunit) chroman-2,4-dione, denoted as compound 1;
[0046] Chromone-substituted 2-hydroxypyrrole derivatives ((E)-3-(7-benzoyl-5-hydroxy-5-(p-tolyl)-2,3-dihydro-1H-pyrrolo[1 , 2-a] the synthetic method of imidazole-6-(5H)-subunit) chroman-2,4-dione), concrete steps are as follows:
[0047] Add 6 mL of 1,4-dioxane as a solvent in a 25 mL round bottom flask, then add p-toluoylglyoxal hydrate, 4-hydroxycoumarin and 2-(imidazolidin-2-ylidene) in sequence -1-phenylethan-1-one, wherein the moles of p-toluoylglyoxal hydrate, 4-hydroxycoumarin and 2-(imidazolidin-2-ylidene)-1-phenylethan-1-one The ratio is 1:1:1, the ratio mol:L of the molar weight of benzoyl formaldehyde derivative (toluoyl formaldehyde hydrate)...
Embodiment 2
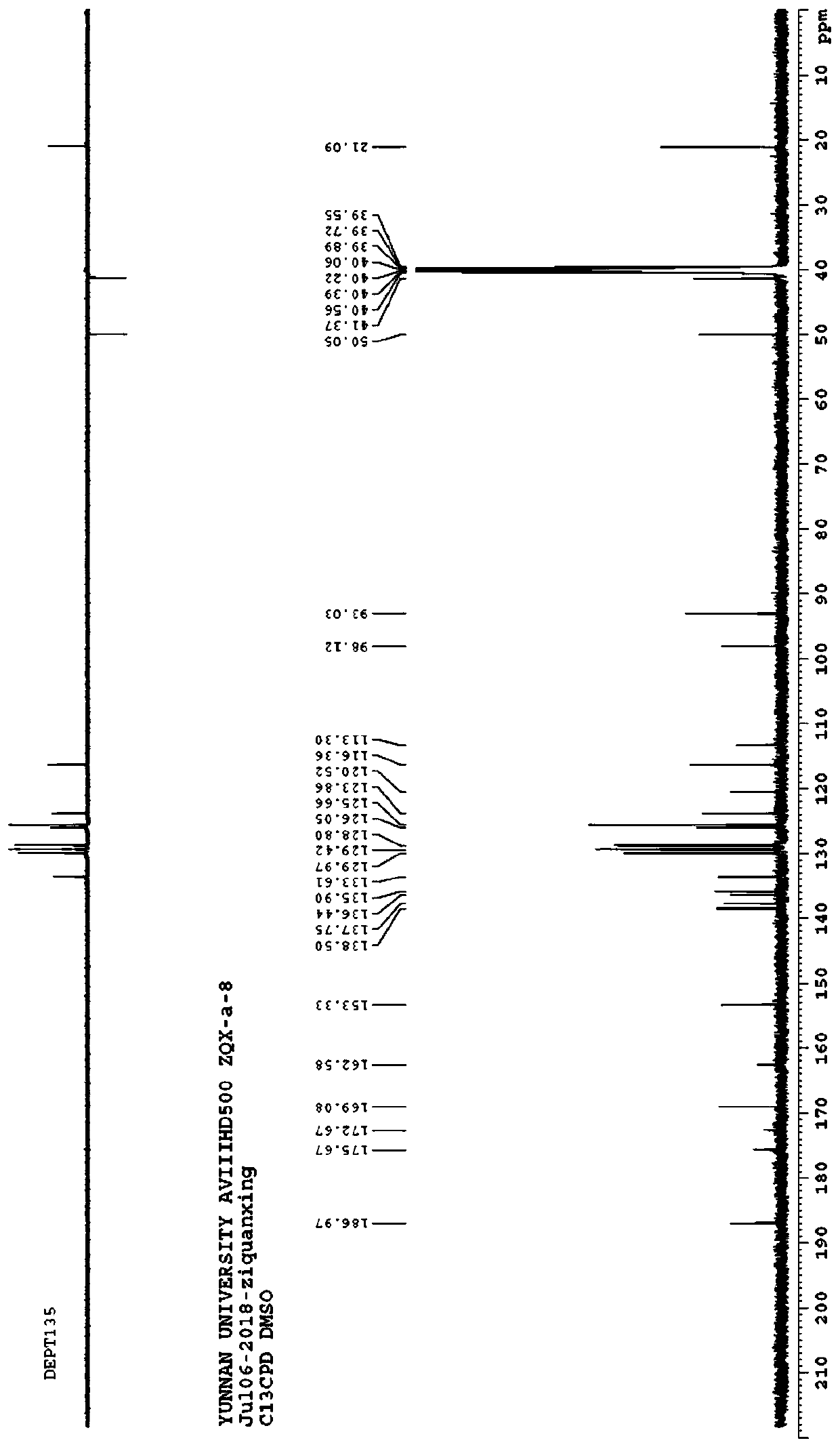
[0054] Example 2: The structural formula of the 2-hydroxypyrrole derivative substituted by the chromone group in this example is shown in the following formula
[0055]
[0056] Named (E)-3-(7-(4-chlorobenzoyl)-5-hydroxy-5-(p-tolyl)-2,3-dihydro-1H-pyrrolo[1,2-a] Imidazole-6-(5H)-ylidene) chroman-2,4-dione, denoted as compound 2;
[0057] Chromone-substituted 2-hydroxypyrrole derivatives ((E)-3-(7-(4-chlorobenzoyl)-5-hydroxy-5-(p-tolyl)-2,3-dihydro-1H - The synthetic method of pyrrolo[1,2-a] imidazole-6-(5H)-ylidene) chroman-2,4-dione), concrete steps are as follows:
[0058] In a 25mL round bottom flask, add 10mL of 1,4-dioxane as a solvent, then add phenylglyoxal, 4-hydroxycoumarin and 1-(4-chlorophenyl)-2-(imidazole Alk-2-ylidene)ethan-1-one, in which phenylglyoxal, 4-hydroxycoumarin and 1-(4-chlorophenyl)-2-(imidazolidin-2-ylidene)ethan-1 -The molar ratio of the ketone is 1:1:1, the ratio mol:L of the molar weight of the phenylglyoxal derivative (phenylglyoxal) to the...
Embodiment 3
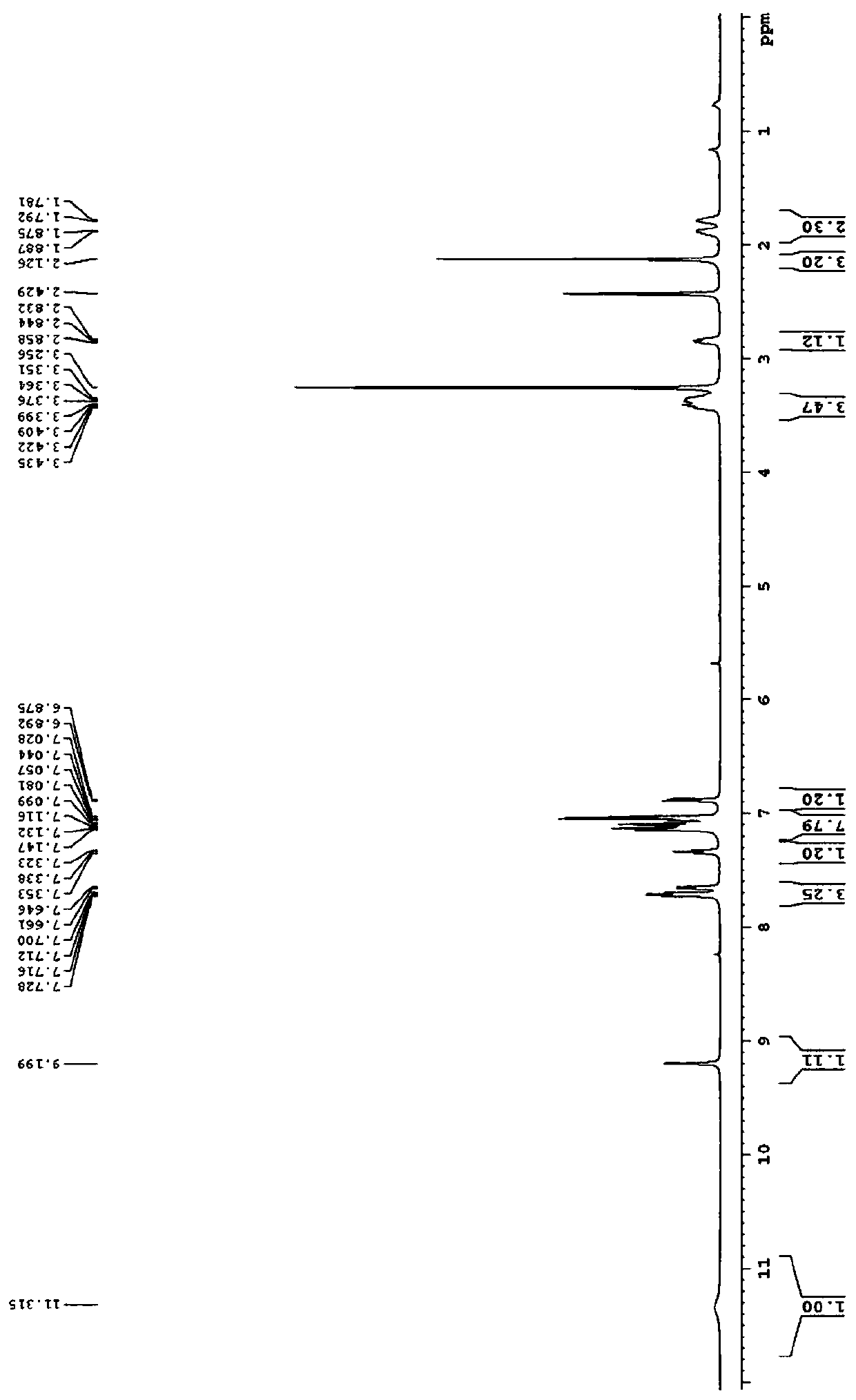
[0065] Example 3: The structural formula of the 2-hydroxypyrrole derivative substituted by the chromone group in this example is shown in the following formula
[0066]
[0067] Named (E)-3-(7-benzoyl-5-hydroxy-5-phenyl-2,3-dihydro-1H-pyrrolo[1,2-a]imidazole-6-(5H)- Subunit)-6-chlorochroman-2,4-dione, denoted as compound 3;
[0068] Chromone-substituted 2-hydroxypyrrole derivatives ((E)-3-(7-benzoyl-5-hydroxy-5-phenyl-2,3-dihydro-1H-pyrrolo[1,2- a] the synthetic method of imidazole-6-(5H)-subunit)-6-chlorochroman-2,4-dione), the specific steps are as follows:
[0069] In a 25mL round bottom flask, add 10mL of 1,4-dioxane as a solvent, then add phenylglyoxal hydrate, 6-chloro-4-hydroxycoumarin and 2-(imidazolidine-2- subunit)-1-phenylethan-1-one, in which phenylglyoxal hydrate, 6-chloro-4-hydroxycoumarin and 2-(imidazolidine-2-ylidene)-1-phenylethanol The molar ratio of -1-ketone is 1:1:1, and the ratio mol:L of the molar weight of phenylglyoxal derivatives (phenylglyoxal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com