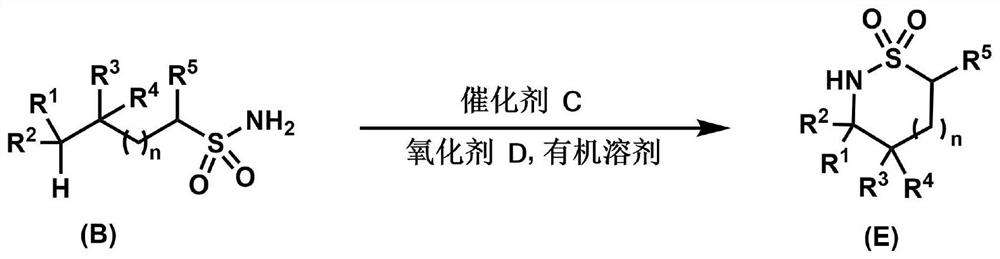
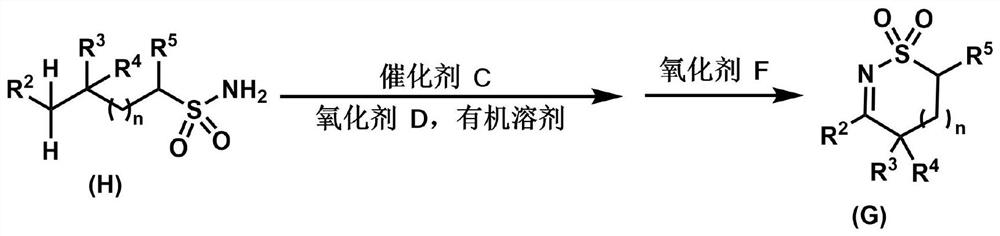
Lactamide compounds and preparation method thereof
A technology for internal sulfonamides and compounds, applied in the field of internal sulfonamide compounds and their preparation, can solve the problems of difficult acquisition, complex catalyst structure, and high price, and achieve the effects of simple reaction operation, reduction of heavy metal residues, and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: Taking phenylpropanesulfonamide as standard substrate, reaction conditions for the synthesis of iron-catalyzed endosulphonamide
[0047]
[0048]
[0049] research:
[0050] Among them, footnote a indicates that the reaction is operated at 60 degrees Celsius; footnote b indicates that molecular sieves are not added to the reaction; wherein [Fe] is an iron salt; the ligand structure is shown in L1-L7 as shown in the table; mol% refers to the relative molar amount, and equiv represents Equivalent, base represents common inorganic base, solvent refers to organic solvent, the volume is 2mL; where DMF is N,N'-dimethylformamide, MeCN is acetonitrile, DCE is 1,2-dichloroethane, 1, 4-dioxane is dioxane, and toluene is toluene. Among them, ligand refers to a multidentate nitrogen ligand, oxidant refers to an oxidizing agent, and yield refers to the total NMR yield of endosulphonamide and endosulphonimide, with s-trimethoxybenzene as the internal standard. ...
Embodiment 2
[0055] 3-(4-Methylphenyl)isothiazolidine-1,1-dioxo[3-(4-Tolyl)isothiazolidine 1,1-dioxide]:
[0056]
[0057] First weigh ferrous perchlorate (5.1mg, 0.02mmol) and ligand L2 (8.2mg, 0.04mmol) into a 4mL reaction bottle, add 1.0mL acetonitrile to dissolve, stir at room temperature for 30 minutes, and in situ complexation When finished, weigh Molecular sieves (50.0mg), iodobenzene pivalate (163.8mg, 0.4mmol) and p-toluenepropanesulfonamide substrate (42.3mg, 0.2mmol) were added to the reaction system, and then 1.0mL of acetonitrile was added to dissolve, and at 80 At ℃, react for 2 h, filter, wash the filter cake with an appropriate amount of saturated sodium bicarbonate, extract the aqueous phase with dichloromethane 3 times (3×10 mL), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, and remove the solvent Afterwards, separated by column chromatography (dichloromethane / petroleum ether=1:1~dichloromethane) to obtain the sulfonamide 3...
Embodiment 3
[0059] 3-(4-Methoxyphenyl)isothiazolidine-1,1-dioxo[3-(4-Methoxyphenyl)isothiazolidine1,1-dioxide]
[0060]
[0061] White solid; yield 86%; 1 H NMR (400MHz, CDCl 3 )δ7.32(d,J=8.8Hz 2H), 6.90(d,J=8.8Hz 2H),4.79–4.63(m,1H),4.50(br s,1H),3.81(s,3H),3.45 –3.32 (m,1H),3.25–3.17(m,1H),2.76–2.68(m,1H),2.44–2.34(m,1H); 13 C NMR (100 MHz, CDCl 3 )δ159.8, 132.0, 127.5, 114.5, 58.0, 55.5, 48.5, 32.4; HRMS(ESI+) calc’d for C 10 h 13 NNaO 3 S[M+Na] + :250.0508, found 250.0513.
[0062] 3-Phenylisothiazolidine 1,1-dioxide
[0063]
[0064] white solid
[0065] 1 H NMR (400MHz, CDCl 3 ( m,1H),2.45–2.34(m,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com