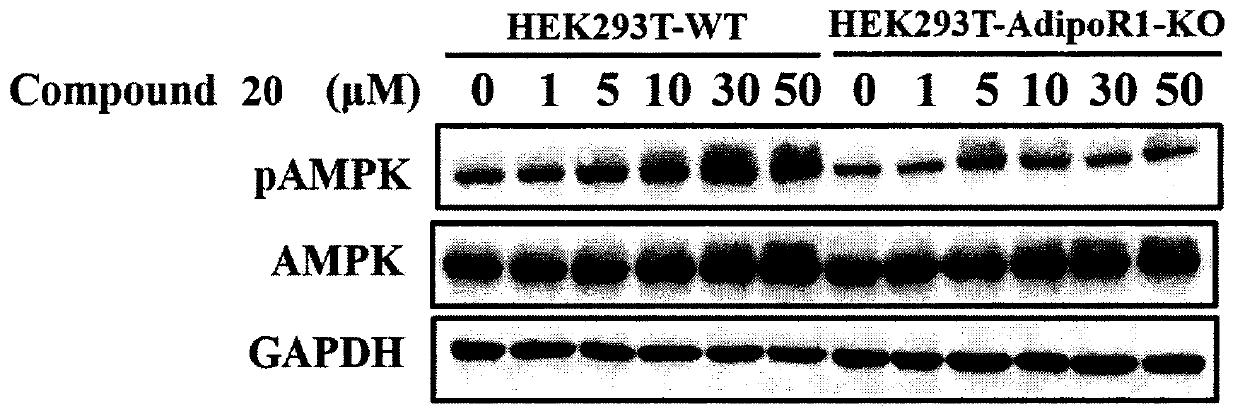
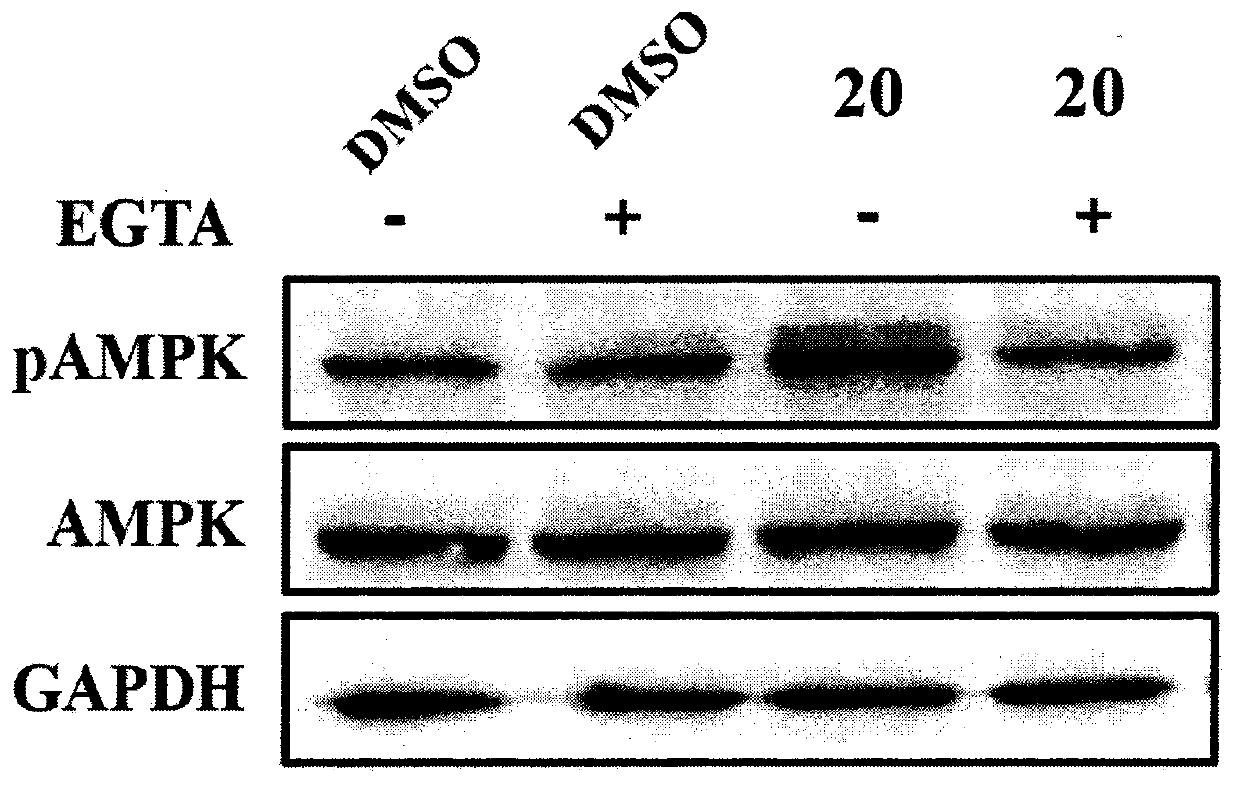
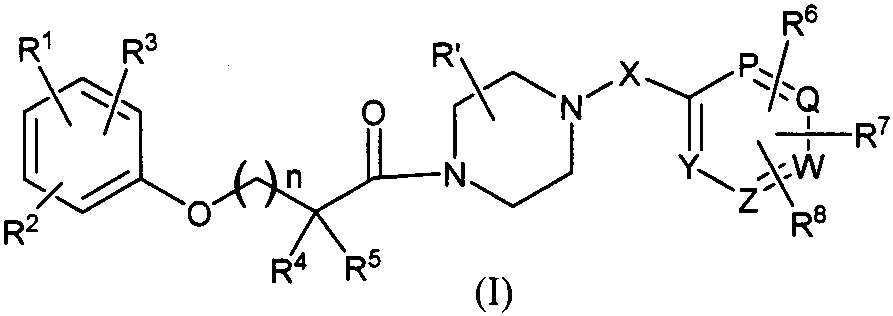
Piperazine adenosine monophosphate activated protein kinase (AMPK) agonist and medical application thereof
A technology of pharmacy and prodrug, applied in the field of piperazine compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] 5-(2,5-Dimethylphenoxy)-1-(1-(4-((4-fluorophenyl)sulfonyl)piperazinyl))-2,2-dimethyl-1- Pentanone (Compound 3)
[0099]
[0100] Compound I-1 (10 g, 40 mmol) was dissolved in dichloromethane (160 mL), and oxalyl chloride (6.77 mL, 80 mmol) and N,N-dimethylformamide (1 mL) were added dropwise under ice-cooling conditions. After reacting for about 5.5 hours, the solvent and unreacted oxalyl chloride were distilled off under reduced pressure to obtain acid chloride. Compound II-1 (11.175g, 60mmol) was dissolved in dichloromethane (100mL), and the above-prepared acid chloride and triethylamine (16.6mL, 120mmol) were added under ice-cooling conditions, and reacted for 12 hours. The reaction system was washed with water (100mL x 3), saturated sodium carbonate solution (100mL x 2), saturated brine (100mL x 2), dried over anhydrous sodium sulfate, the solvent was evaporated under reduced pressure, and the residue was subjected to column chromatography ( Eluent: petroleum e...
Embodiment 2
[0104] 5-(2,5-Dimethylphenoxy)-1-(1-(4-((4-chlorophenyl)sulfonyl)piperazinyl))-2,2-dimethyl-1- Pentanone (compound 1)
[0105] Compound 1 was obtained with reference to the method of Example 1: 1 H NMR (300MHz, CDCl 3 )δ7.64(d, J=8.6Hz, 2H), 7.42(d, J=8.6Hz, 2H), 7.02(d, J=7.5Hz, 1H), 6.68(d, J=7.4Hz, 1H) , 6.58(s, 1H), 3.86(t, J=5.4Hz, 2H), 3.77-3.68(m, 4H), 3.06-2.97(m, 4H), 2.31(s, 3H), 2.14(s, 3H ), 1.78-1.62 (m, 4H), 1.24 (s, 6H). ESI-MS: m / z 515.2 [M+Na] + .
Embodiment 3
[0107] 5-(2,5-Dimethylphenoxy)-1-(1-(4-((4-(trifluoromethyl)phenyl)sulfonyl)piperazinyl))-2,2-di Methyl-1-pentanone (Compound 4)
[0108] Compound 4 was obtained by referring to the method of Example 1: 1 H NMR (300MHz, CDCl 3 )δ7.83(d, J=8.1Hz, 2H), 7.71(d, J=8.2Hz, 2H), 7.02(d, J=7.4Hz, 1H), 6.68(d, J=7.3Hz, 1H) , 6.58(s, 1H), 3.86(t, J=5.2Hz, 2H), 3.81-3.70(m, 4H), 3.06(m, 4H), 2.31(s, 3H), 2.14(s, 3H), 1.79-1.61 (m, 4H), 1.24 (s, 6H). ESI-MS: m / z 549.2 [M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com