Divalent platinum complex and preparation method and application thereof
A platinum complex, complex technology, applied in platinum group organic compounds, platinum group organic compounds, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
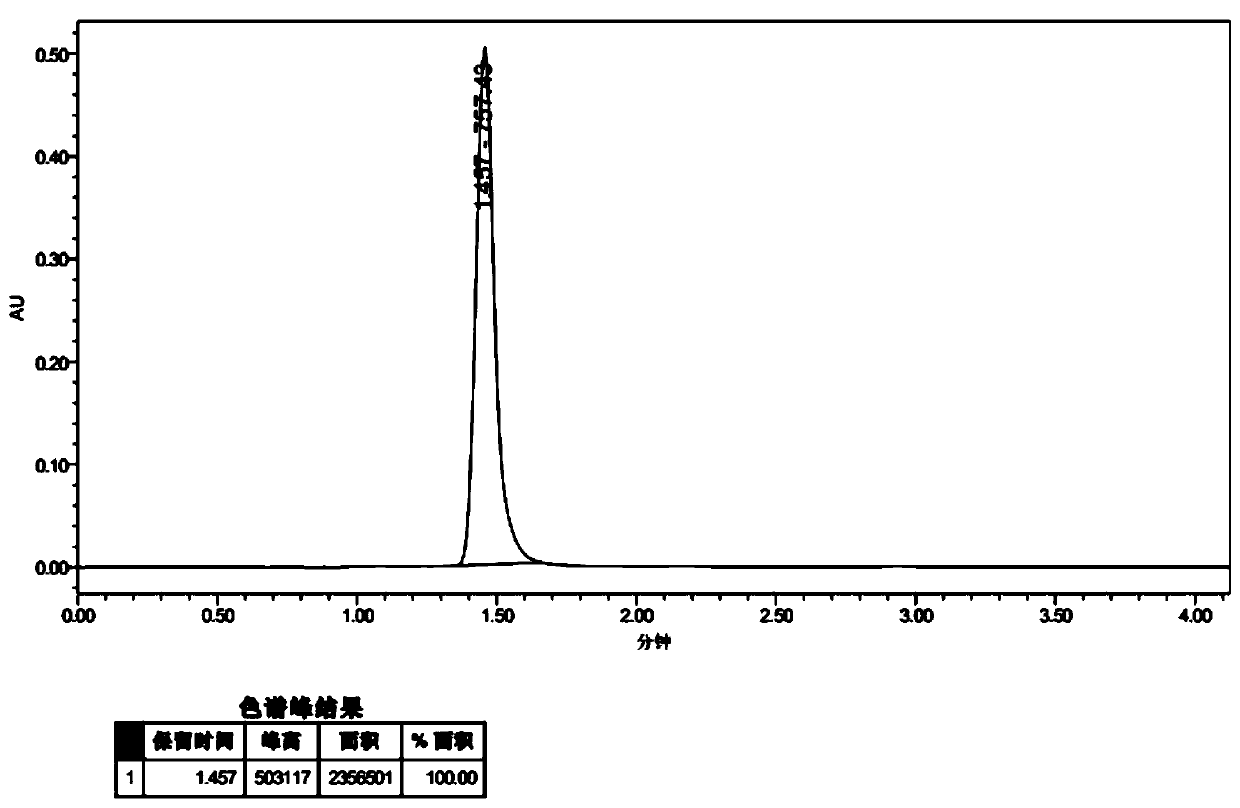
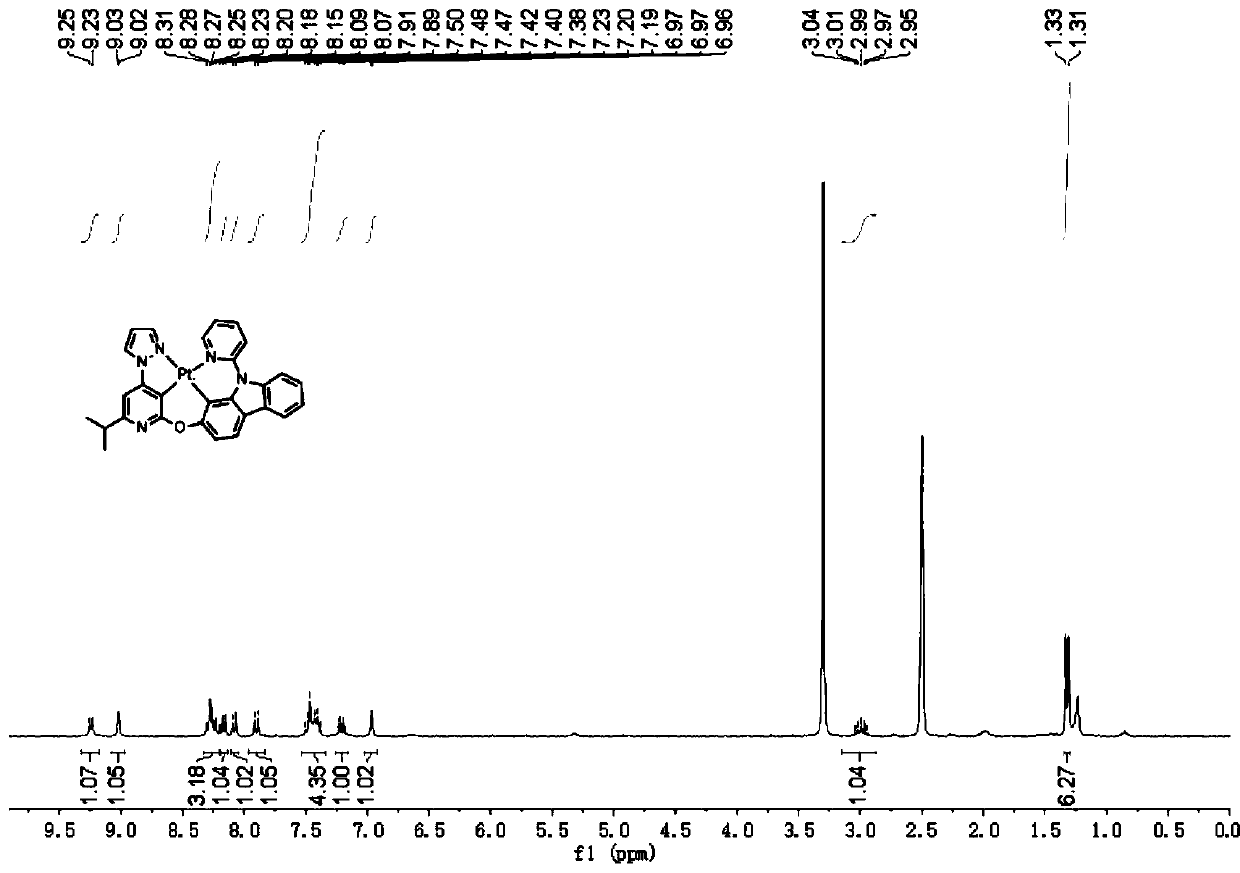
[0069] Embodiment 1 Complex 2 and its preparation
[0070] Synthesis of 2-((6-bromo-4-(1H-pyrazol-1-yl)pyridin-2-yl)oxy)-9-(pyridin-2-yl)-9H-carbazole:
[0071]
[0072] To a sealed 75 mL tube with a magnetic rotor was added 2,6-dibromo-4-(1H-pyrazol-1-yl)pyridine (2.11 g, 7 mmol), 9-(2-pyridyl)-9H-carb Azol-2-ol (1.65g, 6.36mmol), N,N-dimethylglycine (526mg, 5.1mmol), cuprous iodide (121mg, 0.636mmol), cesium carbonate (5.21g, 16mmol) and 1, 4-Dioxane (25 mL), the resulting mixture was bubbled with nitrogen for 10 minutes and then heated to 120° C. and stirred for 48 hours. Cool to room temperature, add water to quench the reaction, extract with ethyl acetate, combine the organic phases, wash with an appropriate amount of saturated aqueous sodium chloride solution and dry over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and the obtained crude product was separated and purified by silica gel column chromatography, and the eluent was pet...
Embodiment 2
[0082] Embodiment 2 Complex 3 and its preparation
[0083] Synthesis of 2-((6-isobutyl-4-(1H-pyrazol-1-yl)pyridin-2-yl)oxy)-9-(pyridin-2-yl)-9H-carbazole L3:
[0084]
[0085] To a sealed 5 mL tube with a magnetic rotor was added 2-((6-bromo-4-(1H-pyrazol-1-yl)pyridin-2-yl)oxy)-9-(pyridin-2-yl) -9H-carbazole (241mg, 0.5mmol), isobutylboronic acid (102mg, 1mmol), tetrakistriphenylphosphopalladium (18mg, 0.015mmol), potassium phosphate (200mg, 0.75mmol) and toluene (5mL), to obtain The mixture was bubbled with nitrogen for 10 min and then heated to 100 °C and stirred overnight. Cool to room temperature, add water to quench the reaction, extract with ethyl acetate, combine the organic phases, wash with an appropriate amount of saturated aqueous sodium chloride solution and dry over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and the obtained crude product was separated and purified by silica gel column chromatography, the eluent was petrol...
Embodiment 3
[0089] Embodiment 3 Complex 4 and its preparation
[0090] Synthesis of 2-((6-trimethylphenyl-4-(1H-pyrazol-1-yl)pyridin-2-yl)oxy)-9-(pyridin-2-yl)-9H-carbazole L4:
[0091]
[0092] To a sealed 5 mL tube with a magnetic rotor was added 2-((6-bromo-4-(1H-pyrazol-1-yl)pyridin-2-yl)oxy)-9-(pyridin-2-yl) -9H-carbazole (168mg, 0.35mmol), 2,4,6-trimethylphenylboronic acid (115mg, 0.7mmol), tris(dibenzylideneacetone) dipalladium (10mg, 0.0105mmol), tricyclohexyl Phosphine (4mg, 0.014mmol), cesium carbonate (228mg, 0.7mmol) and dioxane (1mL), the resulting mixture was bubbled with nitrogen for 10 minutes and then heated to 80°C and stirred overnight. Cool to room temperature, add water to quench the reaction, extract with ethyl acetate, combine the organic phases, wash with an appropriate amount of saturated aqueous sodium chloride solution, and dry over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and the obtained crude product was separated a...
PUM
| Property | Measurement | Unit |
|---|---|---|
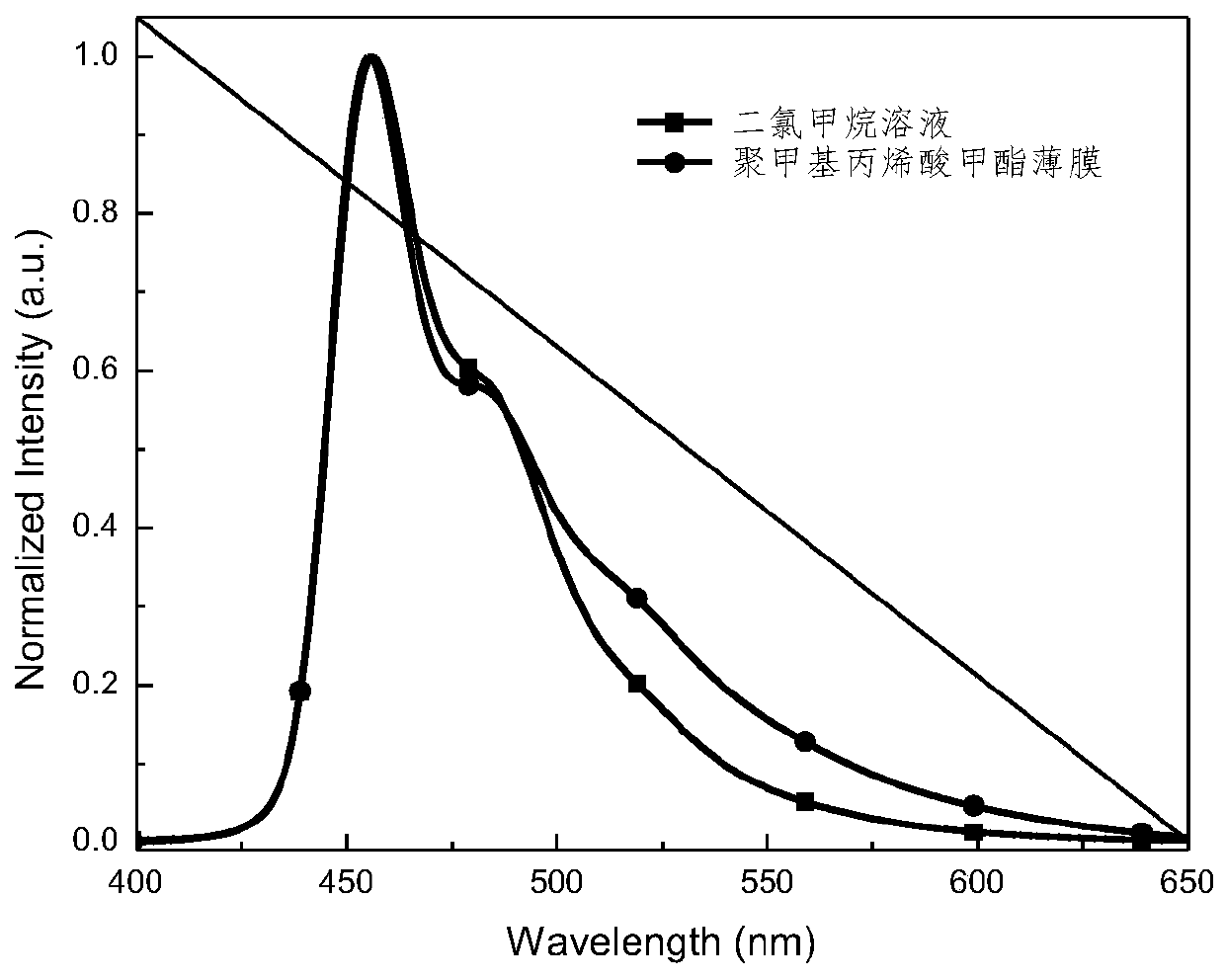
| Luminous wavelength | aaaaa | aaaaa |
| Emission peak | aaaaa | aaaaa |
| Half width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com