Composition containing acotiamide hydrochloride and preparation method of composition
A technology of acotiamide hydrochloride trihydrate and acotiamide hydrochloride, which is applied in the field of compositions containing acotiamide hydrochloride and its preparation, can solve the problems of easy water loss, high ratio, and slow dissolution rate, etc. Achieve the effects of avoiding crystal transformation, excellent comprehensive performance, and accelerating disintegration speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
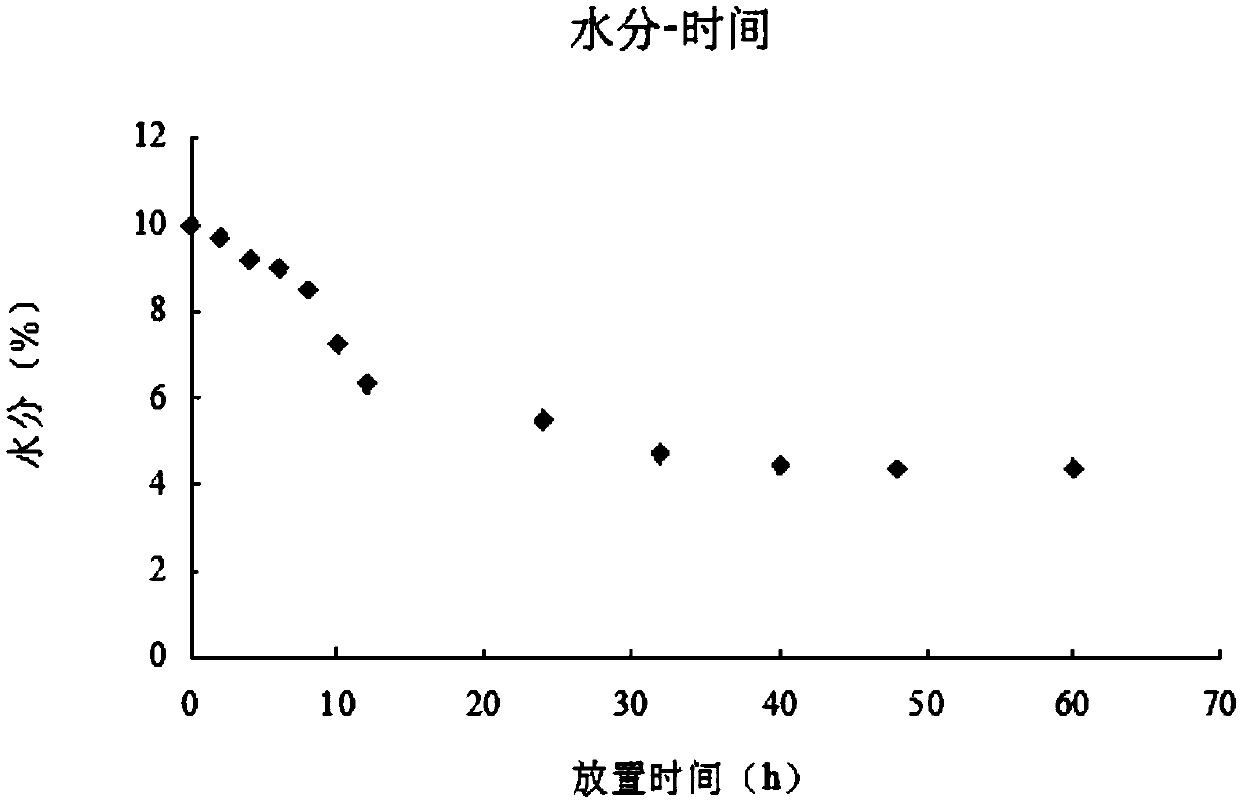
[0070] To study the influence of temperature and humidity on the stability of the crystal form of acotiamide hydrochloride, the raw material drug, that is, the active ingredient, was respectively placed under the humidity conditions of 18% RH and 35% RH, and the change of its moisture content was investigated.
[0071] (1) 20℃ / 18%RH Moisture Inspection
[0072] Under the condition of 20°C / 18%RH, test its moisture content at different storage times, the results are shown in Table 1, and the corresponding scatter plots are shown in figure 1 shown.
[0073] Table 1 20℃ / 18%RH moisture survey data
[0074] Placement time (h) Moisture (%) 0 9.98 2 9.65 4 9.18 6 8.98 8 8.51 10 7.24 12 6.35 24 5.47 32 4.72 40 4.39 48 4.35 60 4.38
[0075] The data show that acotiamide hydrochloride trihydrate is an unstable crystal form under lower humidity conditions; as the standing time prolongs, two molecules of water ar...
Embodiment 2
[0090] Particle size of raw materials: Acotiamide hydrochloride is slightly soluble in water, and almost insoluble in pH 1.0 hydrochloric acid medium, which causes the problem of low solubility and slow dissolution rate of acotiamide hydrochloride tablets.
[0091] Table 4 raw materials
[0092] Drugs / Excipients Dosage (g) Acotiamide hydrochloride trihydrate* 100* lactose 78 Microcrystalline Cellulose PH101 32 Hydroxypropyl Cellulose 10 Low-substituted hydroxypropyl cellulose 10 inner plus +10 extra silica 7.5 Magnesium stearate 2.5 Film Coating Premix 7.5 production 257g / 1000 pieces
[0093] Note: * is the amount of feed calculated by anhydrous
[0094] Weigh the prescribed amount of acotiamide hydrochloride trihydrate, filler, glidant, disintegrant (internal added part), and adhesive and place it in a three-dimensional mixer and mix for 20 minutes. The above mixture is granulated in a dry granulator wit...
Embodiment 3
[0102] Addition method of disintegrant: Investigate the methods of all internal addition, all external addition and internal and external addition of disintegrant (internal and external addition ratio 1:1).
[0103] The raw materials used are shown in Table 6.
[0104] Table 6 raw materials
[0105] Drugs / Excipients Dosage (g) Acotiamide hydrochloride trihydrate* 100* lactose 78 Microcrystalline Cellulose PH101 32 starch 10 Low-substituted hydroxypropyl cellulose 20 talcum powder 7.5 Magnesium stearate 2.5 Film Coating Premix 7.5 production 257g / 1000 pieces
[0106] Note: * is the amount of feed calculated by anhydrous
[0107] Group 1:
[0108] Addition of disintegrant: mechanically pulverize acotiamide hydrochloride trihydrate, and weigh the prescribed amount of active ingredients, fillers, glidants, disintegrants, and binders and place them in three-dimensional mixing Mix in the machine for 20 minutes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com