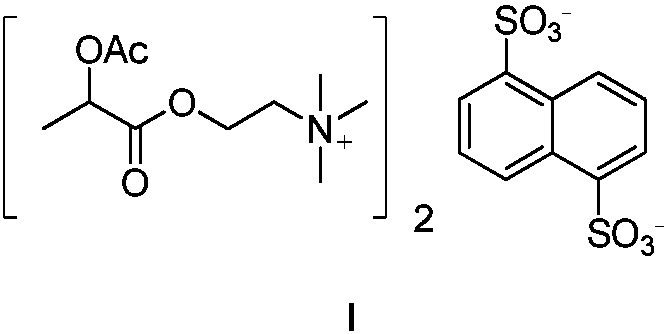
Industrial preparation method of aclatonium napadisilate
A technology of ethacylcholine naphthalene disulfonate and acetyllactoylcholine, which is applied in the field of industrial preparation of acetyllactone naphthalene disulfonate, and can solve the problems of difficult acquisition of raw materials, unfriendly environment, poor stability of intermediates, etc. , to avoid the use of volatile strong corrosive reagents, improve product purity, and reduce unit operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
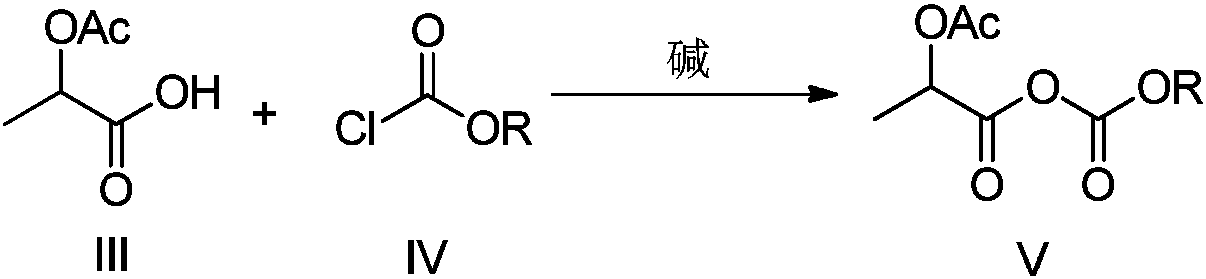
Method used
Image
Examples
Embodiment 1
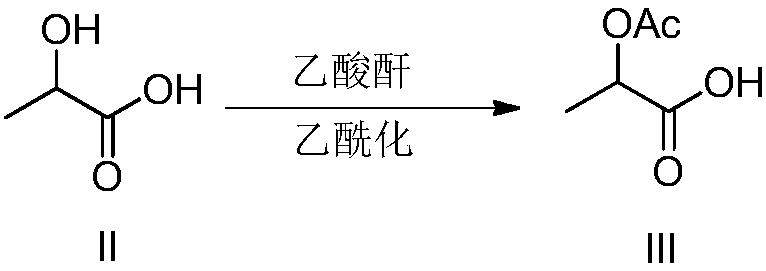
[0032] Step 1): Small-scale preparation of acetolactic acid catalyzed by hydrochloric acid - add 200g of lactic acid (85% aqueous solution, 1.00 equivalent) and 5mL of concentrated hydrochloric acid into a 1L three-necked flask and stir mechanically, reduce the reaction to 0-10°C, and control the reaction temperature At 10-20°C, add 360g (1.90 equivalent) of acetic anhydride dropwise for 45 minutes. After the dropwise addition, transfer the reaction solution to a 1L single-necked bottle, slowly raise the temperature to 90-100°C and concentrate under reduced pressure until the weight of the residue is about 250g. , to obtain acetolactic acid with a yield of about 100%, which is directly used in subsequent reactions.
Embodiment 2
[0034] Step 1): Small-scale preparation of acetolactic acid catalyzed by improved hydrochloric acid - add 200g of lactic acid (85% aqueous solution, 1.00 equivalent) to a 1L three-neck flask, reduce the reaction to 0-10°C, add 1mL of acetyl chloride, dropwise After stirring for 5 minutes, continue to add 360 g (1.90 equivalents) of acetic anhydride dropwise. Control the reaction temperature at 10-20°C for 25 minutes. Concentrate under pressure until the weight of the residue is about 250g to obtain acetolactic acid with a yield of about 100%, which is directly used in subsequent reactions.
Embodiment 3
[0036] Step 1): small-scale preparation of acetolactic acid catalyzed by sulfuric acid—similar to Example 1, add 200 g of lactic acid (85% aqueous solution, 1.00 equivalent) and 5 mL of concentrated sulfuric acid into a 1 L three-necked flask, stir mechanically, and reduce the reaction to 0 At ~10°C, add 360g (1.90 equivalent) of acetic anhydride dropwise for 30 minutes. After the dropwise addition, transfer the reaction solution to a 1L single-necked bottle, slowly raise the temperature to 90-100°C and concentrate under reduced pressure until the weight of the residue is about 270g, that is Acetolactic acid (containing sulfuric acid) is obtained with a yield of about 100%, which can be directly used in subsequent reactions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com